Abstract
Quinolones, in addition to their antibacterial activities, act as immunomodulators. Osteopontin (OPN), a member of the extracellular matrix proteins, was found to play a role in the immune and inflammatory response. We found that quinolones significantly enhanced OPN secretion, namely, garenoxacin (220%), moxifloxacin (62%), gatifloxacin (82%), sparfloxacin, (79%), and sitafloxacin (60%). Enhancement of OPN secretion was shown to be due to the effect of quinolones on the OPN gene promoter activity. We also examined the role of quinolones on apoptosis and found that sparfloxacin decreased the late apoptosis of A549 cells, but garenoxacin did not show the antiapoptotic effect. The antiapoptotic effects of quinolones do not appear to be associated with OPN elevation.
INTRODUCTION
Quinolones are synthetic, broad-spectrum antimicrobial agents widely used in clinical and veterinary medicine. They target two essential bacterial enzymes, DNA gyrase and topoisomerase IV (14). Newly developed quinolones especially possess significant in vivo bactericidal activity, which makes them attractive therapeutic agents for treatment of tuberculosis, community-acquired pneumonia, and other respiratory tract infections (20). In addition to the bactericidal property, fluoroquinolones (FQs) have been found to elicit an immunomodulatory effect (10).
A number of reports have described the inhibitory effect of FQs on cytokine production. Gatifloxacin (GAT) reduced interleukin-8 (IL-8) release from unstimulated cells of the prostatic cancer cell line PC-3 as well as peptidoglycan-, Mycoplasma hominis-, phorbol ester (phorbol myristate acetate [PMA])-, and tumor necrosis factor alpha (TNF-α)-stimulated PC-3 cells but did not significantly reduce the basal level of TNF-α and IL-6 (34). Moxifloxacin (MFX) inhibited IL-8, TNF-α, and IL-1β production in THP-1 cells and in monocytes when preincubated with MFX and stimulated with lipopolysaccharide (LPS) (38). Another report suggested that ciprofloxacin (CIP) may have an immunomodulatory effect on septic patients by attenuating the proinflammatory response, thus decreasing TNF-α, IL-6, IL-1β, and IL-8 levels in patients' serum (15). Levofloxacin at concentrations of 100 μg/ml and higher was found to dose dependently reduce the IL-6 and IL-8 levels in TNF-α-stimulated NL20 human bronchial epithelial cells, but lower concentrations did not alter the studied cytokines (35). Elevated levels of IL-1β, IL-6, and TNF-α in patients with nonbacterial prostatitis became undetectable after treatment with sparfloxacin (SPX) (40).
Several studies attempted to elucidate the signaling pathways and transcription factors that regulate the quinolone-induced cytokine modulation. In A549 cells, IL-1β increased the activities of early intracellular signaling molecules, extracellular signal-regulated kinases 1 and 2 (ERK1/2), phosphorylated Jun N-terminal protein kinase (p-JNK), and NF-κB, whose activities were abrogated by MFX (39). Likewise, in human blood neutrophils, it has been reported that grepafloxacin strongly phosphorylates p38 mitogen-activated protein (MAP) kinase (MAPK) but not p44/42 MAPK or JNK (25).
Osteopontin (OPN), a member of the extracellular matrix proteins, is a multifunctional phosphoprotein that is synthesized by a variety of immune and nonimmune cells (31). Basically, there are three major functions of OPN: involvement in tumorigenesis and metastasis, in mineral metabolism and bone remodeling, and in immune reaction and host defense (36). Because the primary structure and distribution of posttranslational modification are highly conserved among species, it is conceivable that OPN plays an indispensable role in the immune system (8, 9, 32). Within the immune system, OPN is a cytokine secreted by activated T cells, NK cells, dendritic cells, and macrophages (37). In the lung, OPN is expressed by alveolar macrophages and bronchial epithelial cells, the passive physiological barrier of the innate immune system (5, 11, 21). Recently, there is increasing evidence that OPN exists in two isoforms, secreted (sOPN) and/or intracellular (iOPN) protein (36). While sOPN affects the target cell functions by binding to their cell receptors, iOPN binds to MyD88, the downstream protein of the Toll-like receptor (29, 30). Furthermore, it has been reported that OPN binding to CD44v downregulated IL-10 production in macrophages, leading to inhibition of the Th2 immune response, but binding to αvβ3 integrin receptor led to the expression of IL-12, facilitating the Th1 response (2).
When hosts are insulted by infections, Th1 immunity plays a central role in the elimination of microorganisms, so it is understandable that elevated plasma OPN levels have been found to be associated with tuberculosis and other lung inflammatory diseases (26). OPN deficiency was found to be associated with dissemination of mycobacterial disease, and the expression of OPN correlated with an effective immune and inflammatory response and contributed to resistance against mycobacteria in rodents as well as in human (23, 24). OPN also contributed to protection against rotavirus (28), herpes simplex virus type 1 (2, 7), Listeria monocytogenes (2), and Plasmodium falciparum (19). These reports suggest that OPN plays a role in defense mechanisms against invading microorganisms, including viruses, bacteria, and protozoa.
However, until now, the effect of quinolones on OPN expression in human lung epithelial cells remained to be elucidated. A recent report demonstrated that a human lung type II epithelial cell line (A549) is a suitable model to study host defense cellular responses (18). Therefore, in this study we chose this experimental model to investigate the immunomodulatory effect of CIP, garenoxacin mesylate hydrate (GRN), MFX, GFL, SPF, and sitafloxacin (STF). The antibiotics chosen were quinolones, which possess activities against various infections. This is the first report showing that quinolones enhance OPN production in lung epithelial cells. Because OPN is thought to elicit antiapoptotic effects (6, 17), we investigated whether quinolone-induced OPN production may increase survival in A549 cells.
MATERIALS AND METHODS
Cells.
The human A549 alveolar epithelial cell line was obtained from Riken Cell Bank (Tsukuba, Japan). A549/OPN-luc cells were established by cotransfection of pOPN1-luc (42) with puromycin resistance vector pPUR (Clontech, Mountain View, CA) at a molar ratio of 5:1, followed by selection in the presence of 1 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO). The cells were maintained in Ham's F-12 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS; Gibco-Invitrogen, Carlsbad, CA) at 37°C in a humidified incubator containing 5% CO2 in air. For the experiments, subconfluent cultures were harvested by brief trypsinization (trypsin-EDTA solution; Nacalai Tesque, Kyoto, Japan) and resuspended in Ham's F-12 medium supplemented with 2% FBS. Cell viability was determined by trypan blue staining (Sigma-Aldrich, St. Louis, MO).
Antibiotic preparation.
CIP (Wako Pure Chemical Industries, Osaka, Japan) and GRN (kindly provided by Taisho Toyama Pharmaceutical, Tokyo, Japan) were dissolved in dimethyl sulfoxide at a concentration of 30 mg/ml, MFX (Santa Cruz, CA) was dissolved in distilled water at a concentration of 10 mg/ml, and GAT, SPX, and STF (Hokkaido University) were dissolved in 0.1 N NaOH at a concentration of 10 mg/ml.
Determination of OPN protein levels.
To measure the OPN levels in the culture supernatants, A549 cells were plated in triplicate at 1 × 104 cells per well in 96-well plates. After 24 h of incubation, the cells were washed twice with serum-free Ham's F-12 medium, and fresh serum-free medium was added. Cells were treated by CIP, GRN, MFX, GAT, SPX, and STF at the indicated concentrations. After further incubation for 48 h, the culture supernatant was harvested and stored at −20°C. The OPN protein levels were measured by a human osteopontin Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
Cell viability.
To measure the effect of the quinolones on cell viability and proliferation, A549 cells were plated in triplicate at 1 × 104 cells per well in 96-well plates. At 24 and 48 h after the addition of the antibiotics, a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Dojindo, Kumamoto, Japan) was performed according to the manufacturer's instructions. Similarly treated wells without cells served as blanks.
Determination of OPN promoter activity.
A549/OPN-luc cells were treated the same as for ELISA. For the luciferase assay, at 24 and 48 h after the treatment, cells were washed twice with phosphate-buffered saline (PBS) and then lysed with a luciferase cell culture lysis reagent (Promega, Madison, WI). The luciferase assay was done with a luciferase assay system (Promega) according to the manufacturer's instructions, and the luciferase activities were measured using a Mithras LB 940 microplate luminometer (Berthold Technologies, Oak Ridge, TN).
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was performed to examine the effect of quinolones on the expression of OPN mRNA. A549 cells were plated at 1 × 106 in a 6-well plate, and after 24 h, the cells were washed twice with serum-free medium. Fresh serum-free medium and quinolones were added at a final concentration of 30 μg/ml, and GRN was added at a concentration of 1, 3, 10, or 30 μg/ml. The cells were incubated for a further 48 h. Cells were lysed with TRIzol reagent (Invitrogen), and total RNA was extracted according to the manufacturer's instructions. After treatment with RNase-free DNase (Promega), the DNA-free RNA (250 ng) was used for synthesis of the first-strand cDNA at 42°C for 60 min using Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time quantitative PCR using Power SYBR green PCR master mix was conducted for 40 cycles at 95°C for 15 s and at 60°C for 1 min in a 96-well format on an ABI StepOne real-time PCR system (Applied Biosystems). Primer sequences were as follows: OPN forward, 5′-ACTCGTCTCAGGCCAGTTG-3′; OPN reverse, 5′-CGTTGGACTTGGAAGG-3′; GAPDH forward, 5′-TGATGACATCAAGAAGGTGG-3′; and GAPDH reverse, 5′-TCCTTGGAGGCCATGTGGGC-3′.
shRNA transfection.
To diminish OPN expression, A549 cells were transfected with short hairpin RNA (shRNA) targeting OPN or control shRNA (Santa Cruz Biotechnology, Inc.) with Effectene transfection reagent (Qiagen, Valencia, CA) for 48 h. In another setting, cells were transfected with shRNA targeting OPN for 24 h and then washed twice with serum-free medium. The 24-h-conditioned medium from nontreated cells or cells treated with 30 μl/ml of GRN or SPX was added, and the cells were cultured for a further 24 h. Cells were then analyzed by fluorescence-activated cell sorting (FACS).
Annexin V/7-AAD staining and flow cytometry.
A549 cells and cell supernatant were harvested and washed twice with PBS and resuspended in binding buffer containing 10 mM HEPES-NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2. A staining mixture consisting of 5 μl annexin V-phycoerythrin and 5 μl 7-aminoactinomycin (7-AAD) (BD Biosciences Pharmingen) was added to 100 μl of cell suspension (1 × 106 cells/ml). Cells were incubated in the dark for 15 min at room temperature and then analyzed on a FACSDiva flow cytometer. Data were analyzed using FlowJo software (FlowJo, Inc.). The cells in early apoptosis were considered to be annexin V positive and 7-AAD negative. Positivity for both annexin V and 7-AAD indicated late apoptosis.
Statistical analysis.
Results are expressed as means ± standard errors of the means. The statistical difference was determined by two-sided Student's t test, and the correlation between OPN promoter activity and OPN secretion was obtained using simple regression analysis by Statcel2 software (OMS, Tokyo, Japan). A difference with a P value of <0.05 was considered significant.
RESULTS
Quinolones enhance OPN release from A549 alveolar epithelial cells.
The A549 cell line, which secretes OPN, serves as a good model to study drug-induced changes in cytokine secretion (13). We found that treatment of the cells with CIP at 30 μg/ml induced a minimal elevation of OPN secretion, while GRN dramatically increased the OPN level in a dose-dependent manner. Treatment with 1 μg/ml to 30 μg/ml of GRN resulted in a 60 to 220% enhancement of OPN secretion. MFX and SPX at doses of 3 μg/ml to 30 μg/ml caused significant OPN enhancements from 23 to 62% and 22 to 79%, respectively. Similarly, 38 to 82% and 23 to 60% elevations of OPN levels were induced by the addition 10 μg/ml to 30 μg/ml of GAT and STF (Fig. 1).
Fig 1.
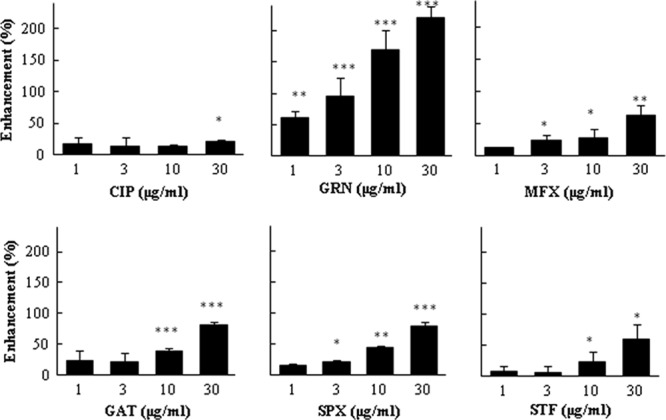
Effect of quinolones on OPN release. A549 cells were plated at 1 × 104 in 96-well plates, and after 24 h, the cells were washed twice with serum-free medium. Fresh serum-free medium and drugs at final concentrations of 1, 3, 10, and 30 μg/ml were added. The cells were incubated for a further 48 h, and the OPN concentrations in the cell culture supernatant were measured by ELISA, as described in Materials and Methods. OPN secretion was normalized by the cell viability optical density (570 to 630 nm) value of the MTT assay and expressed as percent increase above control. Controls are the wells treated only with solvent. The results are expressed as means ± SDs. Representative data of three independent experiments are shown. ***, P < 0.001 compared to the control; **, P < 0.01 compared to the control; *, P < 0.05 compared to the control.
Enhancement of OPN promoter activity.
To investigate the mechanisms of enhancement the OPN secretion, A549/OPN-luc cells, which stably express OPN promoter/luciferase, were established. Thus, luciferase expression of A549/OPN-luc cells served as a parameter to monitor the level of OPN transcription. CIP at 30 μg/ml induced only a 25% elevation of OPN transcription. In contrast, even 24 h cell exposure to 1 μg/ml to 30 μg/ml of GRN for 24 h induced strong, dose-dependent OPN gene activation, reaching 47 to 160%. A similar finding was observed after 48 h. Enhancement of OPN gene promoter activation was observed with increasing concentrations of MFX, GAT, SPX, and STF. However, the change was not so striking compared to that for GRN (Fig. 2). We also examined the association between OPN promoter activation and OPN secretion and found a statistically significant correlation for GRN, GAT, MFX, and STF (Table 1).
Fig 2.
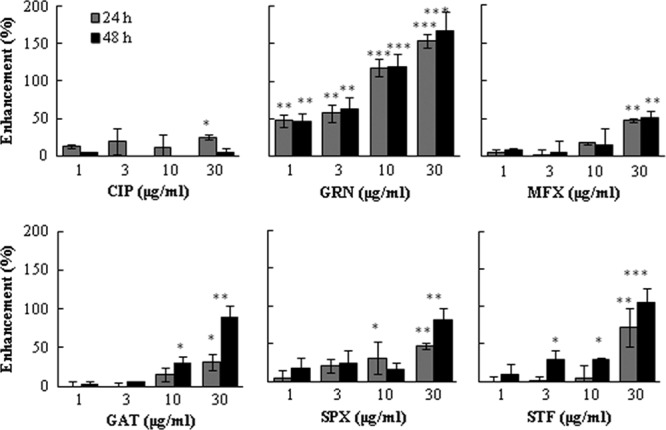
Effect of quinolones on OPN promoter activity. A549 cells were plated at 1 × 104 in 96-well plates, and after 24 h, the cells were washed twice with serum-free medium. Fresh serum-free medium and drugs at final concentrations of 1, 3, 10, and 30 μg/ml were added. Cells were incubated for a further 24 or 48 h, and the OPN gene promoter activity was measured by luciferase assay, as described in Materials and Methods. The OPN promoter activity was normalized by the cell viability optical density (570 to 630 nm) value of the MTT assay and expressed as percent increase above the value for the control. Controls are the wells treated only with solvent. The results are expressed as means ± SDs. Representative data of three independent experiments are shown. ***, P < 0.001 compared to the control; **, P < 0.01 compared to the control; *, P < 0.05 compared to the control.
Table 1.
Correlation between OPN promoter activity and OPN secretion and maximal serum concentrations of studied quinolones
Effect of quinolones on OPN mRNA expression.
To examine if quinolones alter the expression of OPN mRNA, we performed quantitative reverse transcription-PCR. First, we screened the effects of CIP, GRN, MFX, and SPX at a concentration of 30 μg/ml and found that GRN and MFX significantly enhanced mRNA expression compared to that for the control (Fig. 3A). Next, we analyzed the dose-dependent effect of GRN. When cells were incubated with various concentrations of GRN, we observed the dose-related increase of OPN mRNA expression, with statistical significance achieved at concentrations of 10 and 30 μg/ml (Fig. 3B).
Fig 3.
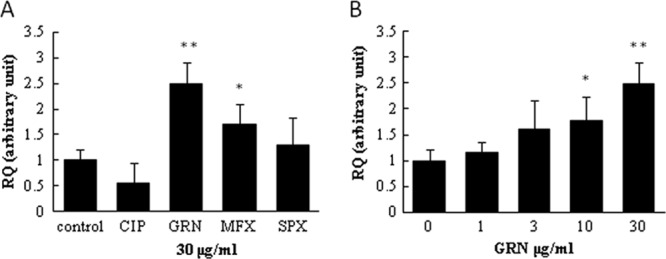
Effect of quinolones on OPN mRNA expression. A549 cells were plated at 1 × 106 in a 6-well plate, and after 24 h, the cells were washed twice with serum-free medium. Fresh serum-free medium and quinolones at a final concentration of 30 μg/ml (A) and GRN at concentrations of 1, 3, 10, and 30 μg/ml (B) were added. The cells were incubated for a further 48 h. Total RNA was isolated, and qRT-PCR was performed as described in Materials and Methods. The results are expressed as means ± SDs. Representative data of three independent experiments are shown. **, P < 0.01 compared to the control; *, P < 0.05 compared to the control.
Effect of quinolones on early and late apoptosis.
To investigate the effect of quinolones on A549 cell apoptosis, we used annexin V/7-AAD double staining for FACS analysis. Treatment of A549 cells with GRN and SPX at a concentration of 30 μg/ml did not affect early apoptosis, but late apoptosis was decreased by SPX (4.42%) and slightly by GRN (9.25%) compared to the control (12.6%). Downregulation of OPN by shRNA resulted in an increase of both early (6.55%) and late (16.7%) apoptosis. When a 24-h supernatant was added to OPN expression-silenced cells, the percentage of apoptotic cells decreased. Surprisingly, the SPX supernatant prevented late apoptosis (5.13%) (Table 2).
Table 2.
FACS analysis showing early and late apoptosis
| Treatmenta | % cells showingb: |
|
|---|---|---|
| Early apoptosis | Late apoptosis | |
| Control | 3.28 | 12.6 |
| DMSO (5%) | 10.9 | 33.8 |
| GRN | 4.82 | 9.25 |
| SPX | 4.74 | 4.42 |
| shRNA | 6.55 | 16.7 |
| Negative-control shRNA | 5.33 | 13.6 |
| shRNA + control supernatant | 4.61 | 12.2 |
| shRNA + GRN supernatant | 4.99 | 14.3 |
| shRNA + SPX supernatant | 4.98 | 5.13 |
A549 cells were plated at 1 × 106 cells per well in 6-well plates. After 24 h of incubation, the cells were washed twice with serum-free Ham's F-12 medium and fresh serum-free medium containing 5% DMSO as a positive control or GRN and SPX at a final concentration of 30 μl/ml, or cells were transfected with shRNA against OPN or negative-control shRNA. Cells were then incubated for a further 48 h and analyzed. Other cells were transfected with shRNA against OPN for 24 h. After the medium was removed, cells were washed twice with serum-free medium; 24-h-conditioned medium from untreated (control supernatant), GRN-treated (30 μg/ml), or SPX-treated (30 μg/ml) cells was added; and cells were incubated for a further 24 h. FACS analysis was performed as described in Materials and Methods. Representative data of three independent experiments are shown.
Cells showing early apoptosis are annexin positive and 7-AAD negative. Cells showing late apoptosis are annexin V positive and 7-AAD positive.
DISCUSSION
In this study, we examined the effect of quinolones on OPN synthesis in A549 human lung epithelial cells. We found that GRN, MFX, GAT, SPX, and STF dose dependently enhanced OPN secretion. On the other hand, CIP at a concentration of 30 μg/ml only slightly enhanced OPN. Since our ELISA system cannot distinguish phosphorylated from nonphosphorylated or cleaved forms of OPN, we could not elucidate which form of OPN was enhanced. To elucidate the mechanism of quinolone-induced OPN elevation, we employed a luciferase assay and found that this elevation occurred at the transcriptional level by activation of the OPN gene promoter. The results of qRT-PCR also revealed that MFX and GRN significantly enhanced OPN mRNA expression, but SPX and CIP did not.
Generally, quinolones are thought to attenuate proinflammatory cytokine synthesis, though there have been reports that CIP caused an increase in IL-2 secretion from PMA-treated human peripheral blood lymphocytes. In contrast, there was no effect on IL-1 release in the same experimental setting (33). Because the effect of quinolones on cytokine synthesis is not consistent and differs by the cell type and the cytokine examined (13), it is tempting to speculate that the cytokine response to drug treatment is cell type specific and influenced by the microenvironment under which cells exist.
In our study, we investigated the effect of quinolone concentrations above and below the maximal concentrations in serum (Cmaxs) (Table 1). The results showed that GAT, SPX, and STF augmented OPN transcription only at concentrations higher than the Cmaxs, but GRN and MFX elicited the ability to enhance OPN even at their Cmaxs. Since OPN is a multifunctional protein, an elevation of OPN synthesis might have a dual effect. OPN can promote protective immunity through the OPN-dependent induction of the proinflammatory cytokine IL-12 and suppression of the anti-inflammatory cytokine IL-10 (2). However, the excessive OPN production might activate various cell growth signals, finally leading to oncogenesis (26, 42). Zhang et al. observed that the OPN gene is transactivated up to 5-fold by Tax protein of human T-cell leukemia virus type 1 (42). However, in our study, the therapeutic concentrations of quinolones did not enhance OPN more than 2-fold. Despite our observation of a quinolone-induced OPN enhancement, it is conceivable that such an elevation would not lead to oncogenesis.
Several studies confirmed the antiapoptotic activities of FQs. Azuma et al. suggested that tosufloxacin delayed programmed cell death via the activation of phosphoinositide 3-kinase (PI3K)/Akt and/or p38 MAPK (3). Since OPN is a downstream effector of PI3K/Akt (27, 42) and OPN elicits an antiapoptotic effect (6, 17), we speculate that the antiapoptotic effect of the quinolones is caused by the enhancement of OPN synthesis. We examined the effect of quinolones on early and late stages of apoptosis by FACS analysis. During early apoptosis, the phosphatidylserine (PS) changes location from the cytosolic leaflet to the outer leaflet of the cell. This event can be detected by annexin V, which binds to the PS (16). The damage of the cell membrane during late apoptosis and necrosis allows insertion of 7-AAD between the tops of successive cytosine and guanine bases (41). We could not observe significant changes in early apoptosis upon quinolone treatment, but SPX considerably decreased late apoptosis in A549 cells. We found that the cell culture medium from SPX-treated cells prevented late apoptosis in OPN shRNA-transfected A549 cells, but GRN-treated and untreated cell supernatants had only minor effects. Among the studied quinolones, GRN exerted the greatest ability to enhance OPN synthesis and did not significantly alter early or late apoptosis in A549 cells. OPN synthesis was also enhanced by SPX treatment but to a lesser extent than by GRN, but an effect of SPX on late apoptosis was clearly observed. This made us conclude that there might be other factors which prevent apoptosis upon quinolone treatment and that the OPN might have only a supportive effect or the OPN enhancement was not sufficient to exert its antiapoptotic property.
Throughout respiratory infections, apoptosis may be beneficial or detrimental for the host (4). In infections in which pathogens exist within the host cells, apoptosis favors the host. Insufficient apoptosis of alveolar macrophages during tuberculosis infection leads to the chronicity and dissemination of the infection. For extracellular infections, apoptosis of the immune inflammatory cells potentiates the viability of the pathogen and promotes the infection (4). Our in vitro finding showed that SPX decreased late apoptosis in A549 cells. The antiapoptotic effect of quinolones on lung epithelial cells in vivo remains to be elucidated.
The quinolones used in our study differ in chemical structure as well as in the ability to influence OPN secretion and apoptosis. The fluorine molecule at the C-6 position, which is present in CIP, MFX, GAT, SPX, STF, and other FQs, is thought to improve the antimicrobial properties of these drugs, but the newly developed GRN is lacking this moiety. GRN has fluorine incorporated through a C-8 difluoromethyl ester linkage (1). Manipulation of the group at position C-8 has also been shown to play a role in broadening the spectrum of activity (12). Among the studied quinolones, only CIP is lacking the substituent at C-8. Our results showed that in contrast to CIP, GRN significantly enhanced OPN production. Therefore, we speculate that the ability of quinolones to enhance OPN production may be associated with the presence of the C-8 substituent.
In conclusion, we found that quinolones enhance OPN synthesis by activation of the OPN gene promoter. The antiapoptotic effects of quinolones do not appear to be associated with OPN elevation. Our study supports the idea that quinolones have immunomodulatory properties.
ACKNOWLEDGMENTS
This work is supported by the Scientific Research Expenses for Health and Welfare program from the Ministry of Health, Labor and Welfare, Japan (to T.H.), and the Science and Technology Research Partnership for Sustainable Development from the Japan Science and Technology Agency, Japan (to Y.S.). This work was supported by collaborative funding from the Research Centre for Zoonosis Control, Hokkaido University.
We are grateful to Shenwei Li, Xiaoguang Li, and Yuko Sato (Tohoku University) for technical assistance. We thank Brent Bell for reading the manuscript.
We declare no competing financial interest.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Andersson MI, MacGowan AP. 2003. Development of the quinolones. J. Antimicrob. Chemother. 51(Suppl. 1):1–11 [DOI] [PubMed] [Google Scholar]
- 2. Ashkar S, et al. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860–864 [DOI] [PubMed] [Google Scholar]
- 3. Azuma Y, Ohura K. 2003. Alteration of constitutive apoptosis in neutrophils by quinolones. Inflammation 27:115–122 [DOI] [PubMed] [Google Scholar]
- 4. Behnia M, Robertson KA, Martin WJ., II 2000. Lung infections: role of apoptosis in host defense and pathogenesis of disease. Chest 117:1771–1777 [DOI] [PubMed] [Google Scholar]
- 5. Brown LF, et al. 1992. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol. Biol. Cell 3:1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burdo TH, Wood MR, Fox HS. 2007. Osteopontin prevents monocyte recirculation and apoptosis. J. Leukoc. Biol. 81:1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cantor H, Shinohara ML. 2009. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat. Rev. Immunol. 9:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen B, et al. 2007. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J. Biol. Chem. 282:19463–19472 [DOI] [PubMed] [Google Scholar]
- 9. Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. 2005. Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem. J. 390:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalhoff A. 2005. Immunomodulatory activities of fluoroquinolones. Infection 33(Suppl. 2):55–70 [DOI] [PubMed] [Google Scholar]
- 11. Diamond G, Legarda D, Ryan LK. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27–38 [DOI] [PubMed] [Google Scholar]
- 12. Dong Y, Xu C, Zhao X, Domagala J, Drlica K. 1998. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnarumma G, et al. 2007. Anti-inflammatory effects of moxifloxacin and human beta-defensin 2 association in human lung epithelial cell line (A549) stimulated with lipopolysaccharide. Peptides 28:2286–2292 [DOI] [PubMed] [Google Scholar]
- 14. Drlica K, Zhao X. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gogos CA, et al. 2004. Comparative effects of ciprofloxacin and ceftazidime on cytokine production in patients with severe sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 48:2793–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koopman G, et al. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420 [PubMed] [Google Scholar]
- 17. Lin YH, Yang-Yen HF. 2001. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J. Biol. Chem. 276:46024–46030 [DOI] [PubMed] [Google Scholar]
- 18. MacRedmond R, Greene C, Taggart CC, McElvaney N, O'Neill S. 2005. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir. Res. 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeno Y, et al. 2006. Osteopontin participates in Th1-mediated host resistance against nonlethal malaria parasite Plasmodium chabaudi chabaudi infection in mice. Infect. Immun. 74:2423–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. 2007. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs 67:2077–2099 [DOI] [PubMed] [Google Scholar]
- 21. Morimoto Y, et al. 2011. Osteopontin modulates the generation of memory CD8+ T cells during influenza virus infection. J. Immunol. 187:5671–5683 [DOI] [PubMed] [Google Scholar]
- 22. Nakashima M, et al. 1995. Pharmacokinetics and tolerance of DU-6859a, a new fluoroquinolone, after single and multiple oral doses in healthy volunteers. Antimicrob. Agents Chemother. 39:170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nau GJ, et al. 2000. Osteopontin expression correlates with clinical outcome in patients with mycobacterial infection. Am. J. Pathol. 157:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nau GJ, et al. 1999. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 67:4223–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niwa M, et al. 2004. P38 MAPK associated with stereoselective priming by grepafloxacin on O-2(−) production in neutrophils. Free Radic. Biol. Med. 36:1259–1269 [DOI] [PubMed] [Google Scholar]
- 26. O'Regan A. 2003. The role of osteopontin in lung disease, Cytokine Growth Factor Rev. 14:479–488 [DOI] [PubMed] [Google Scholar]
- 27. Packer L, et al. 2006. Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis 27:1778–1786 [DOI] [PubMed] [Google Scholar]
- 28. Rollo EE, et al. 2005. The cytokine osteopontin modulates the severity of rotavirus diarrhea. J. Virol. 79:3509–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. 2008. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 105:7235–7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shinohara ML, et al. 2006. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat. Immunol. 7:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sodek J, Ganss B, McKee MD. 2000. Osteopontin. Crit. Rev. Oral Biol. Med. 11:279–303 [DOI] [PubMed] [Google Scholar]
- 32. Sorensen ES, Hojrup P, Petersen TE. 1995. Posttranslational modifications of bovine osteopontin: identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 4:2040–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stunkel KG, Hewlett G, Zeiler HJ. 1991. Ciprofloxacin enhances T cell function by modulating interleukin activities. Clin. Exp. Immunol. 86:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takeyama K, et al. 2007. The 6-fluoro-8-methoxy quinolone gatifloxacin down-regulates interleukin-8 production in prostate cell line PC-3. Antimicrob. Agents Chemother. 51:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsivkovskii R, et al. 2011. Levofloxacin reduces inflammatory cytokine levels in human bronchial epithelia cells: implications for aerosol MP-376 (levofloxacin solution for inhalation) treatment of chronic pulmonary infections. FEMS Immunol. Med. Microbiol. 61:141–146 [DOI] [PubMed] [Google Scholar]
- 36. Uede T. 2011. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 61:265–280 [DOI] [PubMed] [Google Scholar]
- 37. Uede T, Katagiri Y, Iizuka J, Murakami M. 1997. Osteopontin, a coordinator of host defense system: a cytokine or an extracellular adhesive protein? Microbiol. Immunol. 41:641–648 [DOI] [PubMed] [Google Scholar]
- 38. Weiss T, et al. 2004. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob. Agents Chemother. 48:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Werber S, et al. 2005. Moxifloxacin inhibits cytokine-induced MAP kinase and NF-kappaB activation as well as nitric oxide synthesis in a human respiratory epithelial cell line. J. Antimicrob. Chemother. 55:293–300 [DOI] [PubMed] [Google Scholar]
- 40. Yasumoto R, et al. 1995. Seminal plasma cytokines in nonbacterial prostatitis: changes following sparfloxacin treatment. Hinyokika Kiyo 41:771–774 [PubMed] [Google Scholar]
- 41. Zelenin AV, et al. 1984. 7-Amino-actinomycin D as a specific fluorophore for DNA content analysis by laser flow cytometry. Cytometry 5:348–354 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Yamada O, Matsushita Y, Chagan-Yasutan H, Hattori T. 2010. Transactivation of human osteopontin promoter by human T-cell leukemia virus type 1-encoded Tax protein. Leuk. Res. 34:763–768 [DOI] [PubMed] [Google Scholar]


