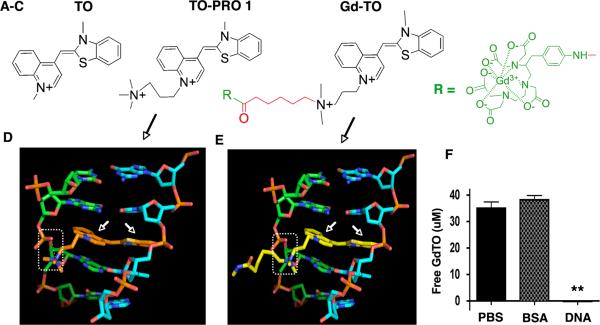
Figure 2.
Structure of Gd-TO and its interaction with DNA. (A–C) From left to right, the structures of thiazole orange (TO), TO-PRO 1 and Gd-TO are shown. TO-PRO 1 and Gd-TO feature quaternary nitrogens, rendering them membrane impermeable. The design of Gd-TO is based on TO-PRO 1, with a quaternary amine, a carboxy terminated linker (red), and a pNH2-Bn-DTPA:Gd chelate reporter (green). (D) The intercalation of the TO-PRO-1 (orange structure, white arrows) with double stranded DNA is compared with (E) the intercalation of Gd-TO (yellow structure, white arrows) with DNA. The quaternary nitrogens on the aliphatic arms of both compounds form salt bridges (dashed rectangles) with the phosphate groups of DNA. (F) Filtration assay showing binding of Gd-TO to DNA but not to albumin (BSA). Gd-TO was incubated with DNA or BSA and the level of free unbound Gd-TO was determined in the filtrate. DNA bound and retained Gd-TO, while BSA and the PBS control did not. ** (p < 0.001).