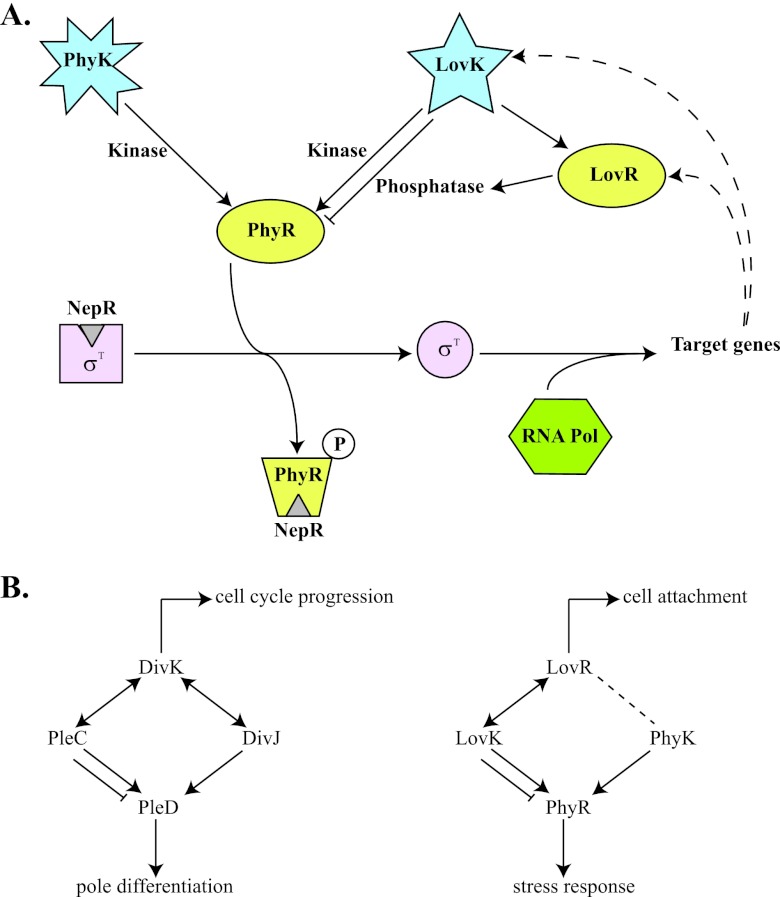
Fig 1.
(A) PhyR-NepR-EcfG-like sigma factor regulatory pathway in C. crescentus. Under nonstress conditions, the anti-sigma factor NepR binds to σT, preventing its association with RNA polymerase. Upon phosphorylation of PhyR by PhyK (activated by stress conditions) or LovK (under conditions that determine low levels of LovR), PhyR∼P binds to NepR so that σT is released and can recruit the RNA polymerase complex for the expression of target genes (dashed lines indicate that lovK and lovR are among the genes activated by σT). When present at high levels, LovR can act as a sink for phosphate and turn LovK into a phosphatase that would keep PhyR unphosphorylated, which would then result in a decrease of expression for σT-dependent genes. (B) Parallel between the DivK-DivJ-PleC-PleD and LovR-LovK-PhyK-PhyR systems. In both cases, a single-domain RR (DivK and LovR), two His kinase-phosphatases (DivJ-PleC and LovK-PhyK), and an RR with output domain that regulate downstream events (PleD and PhyR) are involved. DivK can stimulate the autokinase activity of both DivJ and PleC and determine whether PleC acts as a kinase or a phosphatase. LovR determines if LovK acts as kinase or phosphatase on PhyR, but it is not known whether LovR interacts with PhyK as well. PleD is a substrate for both PleC and DivJ, whereas PhyR is a substrate for both LovK and PhyK. The single-domain RRs not only are a sink for phosphate but can also regulate other processes (cell cycle progression in the case of DivK and cell attachment in the case of LovR).