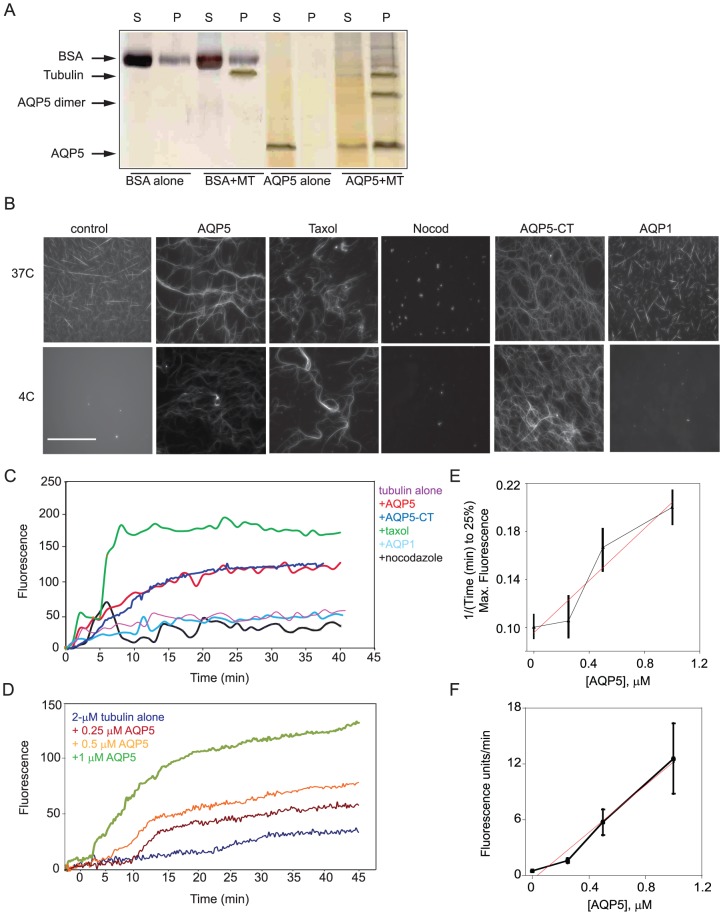
Figure 4. AQP5 purified protein both associated with MTs and altered MT polymerization (all experiments have a minimum of 4 replicates).
A. Using a spin down co-sedimentation assay, polymerized tubulin was primarily in the pellet. AQP5, in the absence of MTs remains in the supernatant, however, after the addition of MTs it was primarily in the pellet. In contrast, as a negative control, BSA was primarily in the supernatant and addition of MTs does not alter its localization. B. Using fluorescence microscopy, changes in MT assembly were visibly apparent. At 37 C, MTs assembled to different extents under all conditions, except in the presence of nocodazole. Upon shifting the temperature to 4 C, MTs disassembled in the tubulin-alone control and in the presence of AQP1. However, MTs remained assembled in the presence of AQP5, taxol and AQP5-CT. (scale bar 100 µM) C. In a fluorescence-based polymerization assay, AQP5 (1 µM) and AQP5-CT, like taxol, increased MT polymerization whereas AQP1 (1 µM) failed to support MT assembly as compared to tubulin alone (2 µM). Nocodazole also inhibited assembly. Taxol (30 µM) and nocodazole (30 µM) were added as positive and negative controls, respectively. D. Addition of increasing concentrations of AQP5 increased the rate and extent of MT assembly in the fluorescence assay. E. Analysis of the fluorescence assembly assay data revealed that the inverse time to 25% assembly, which is largely dominated by nucleation, increased with increasing AQP5 concentration. The slope was 0.35 µM−1 min−1. F. Analysis of the rapid, linear growth phase showed an increasing rate of polymer assembly as a function of AQP5 concentration. The slope was 13 units µM−1 min−1.