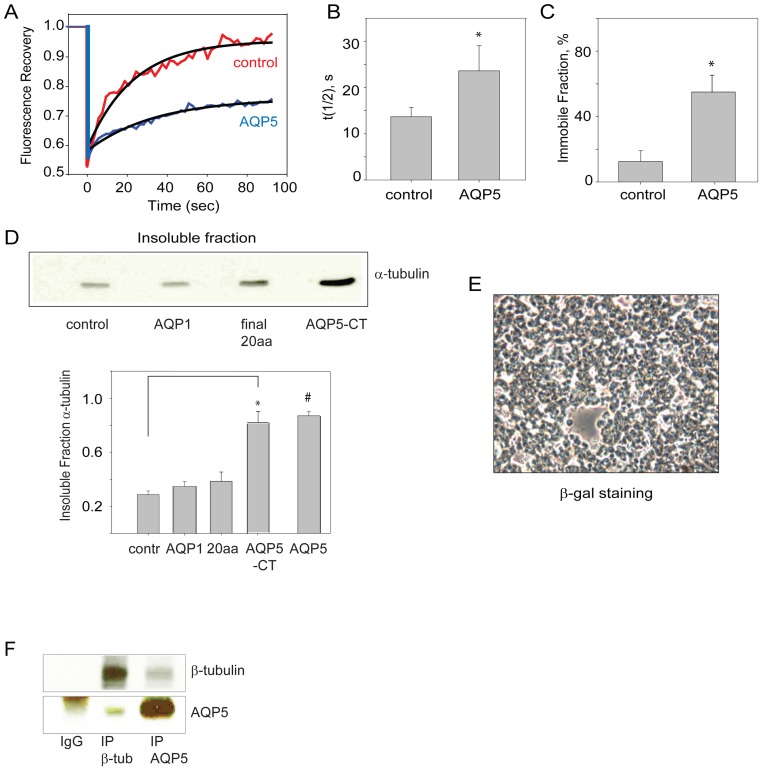
Figure 5. Overexpression of AQP5 led to delayed fluorescence recovery and increased MT stability.
In primary human airway epithelial cells, AQP5 directly binds to tubulin. A. Using FRAP analysis of transduced 16HBE cells, which do not naturally express AQP5, we found that AQP5 delayed fluorescence recovery of GFP-α-tubulin after photobleaching. B. The measured recovery half-life (t1/2) of GFP-α-tubulin increased in cells expressing AQP5 compared to control cells (*, Student's t-Test (ST): p=0.01). C. AQP5-expressing cells also had a significantly increased immobile fraction (*, ST: p<0.01). D. AQP5-CT is sufficient to increase the insoluble fraction of tubulin in HEK cells. Control β-galactosidase, full-length AQP1, the final 20 amino acids of AQP5 or the entire AQP5-CT (carboxyl-terminal 40 amino acids) were transfected into HEK cells using the protein transfection reagent Chariot (Activemotif) and insoluble MT fractions were collected. Full-length AQP5 data was reproduced from Fig. 2 and shown here for direct comparison. Detection of the soluble fraction was minimal in these cells. E. β-galactosidase-staining in control cells confirmed that the transfection efficiency was 90–100%. E. NHBE cells, with immunoprecipitation of b-tubulin, there is pull-down of AQP5, and similarly with immunoprecipitation of AQP5, there is pull-down of b-tubulin. An IgG immunoprecipitation was performed as a control (n=4, per condition).