Figure 2.
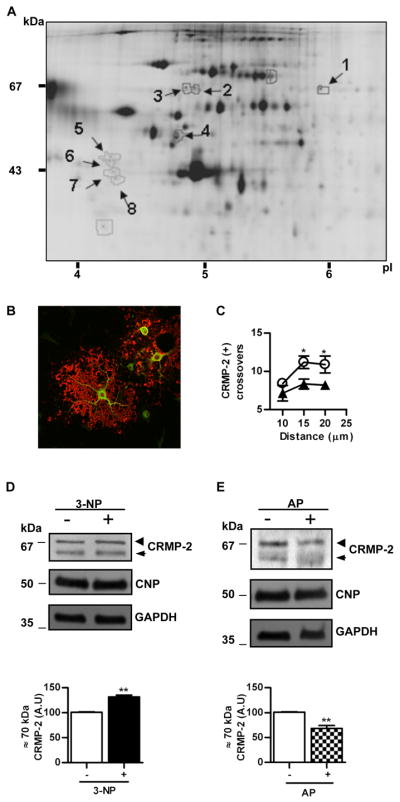
A) Representative 2D-DIGE of proteins from mature OLG homogenates after incubation with 3-NP for 1h. First dimension, isoelectric point (pI); second dimension, molecular mass (kDa). Arrows point spots with a Cy5/Cy3 fluorescent signal change higher or lower than 1.5 selected for identification by tryptic digestion and mass spectrometry. Numbers refer to those proteins identified and listed in Table I (Appendix S3). B) Merge of confocal immunofluorescence of primary OLGs with anti-MBP (red) and anti-CRMP-2 (green). Note the lack of co-localization, as expected from the cytosolic and membrane location of CRMP-2 and MBP, respectively. Scale bar=10μm. C) Plot of the number of CRMP-2 immunoreactive processes that intersect concentric circles at increasing distance from the centre of the cell body in controls (○) or after treatment with 3-NP (▲). *p<0.05, Student’s t test at each distance point. D) Upper panel, representative Western blot of OLG homogenates probed with anti-CRMP-2 in control cells (−) or after treatment with 3-NP (+). Lower panel, bars represent the mean ± SEM of the immunoreactivity of the phosphorylated CRMP-2 isoform (~70 kDa) expressed in arbitrary units (A.U.). E) Upper panel, representative Western blot probed with anti-CRMP-2 of proteins from OLGs after exposure to 3-NP and treated (+) or not (−) with alkaline phosphatase (AP) before SDS-PAGE. Lower panel, bars represent the mean ± SEM of the immunoreactivity of phosphorylated CRMP-2 isoform (~70 kDa) expressed in arbitrary units (A.U.). Membranes were probed with anti-CNP and anti-GAPDH for protein loading control. Arrowheads, phosphorylated CRMP-2 isoform.(~70 kDa); Arrows, unphosphorylated CRMP-2 isoform (~62 kDa). **p<0.001 Student’s t test.
