Abstract
The current study explored the complicated interplay between aphasia and the stress biomarker, cortisol, in left hemisphere (LH) and right hemisphere (RH) stroke patients. Nineteen LH patients and 12 RH patients began the study between one to six months post-stroke and were followed for three months. During this time, language skills were assessed monthly while afternoon salivary cortisol samples were collected biweekly. The LH and RH groups showed improvements in language test scores over the course of three months; however, only naming skills in the RH group appeared to be associated with afternoon salivary cortisol levels. Furthermore, contradicting previous reports regarding laterality and cortisol regulation in humans, the current study found that both LH patients and RH patients exhibited similar afternoon salivary cortisol levels across all time points.
Aphasia may be stressful. Recently a series of studies have indicated that individuals living with aphasia have greater perceived stress and fewer coping resources than typical adults (Laures-Gore, Hamilton, & Matheny, 2007), report greater perceived stress related to a linguistic task than healthy adults (Laures-Gore, Heim, & Hsu, 2007), and report more distress than stroke patients without aphasia (Hilari et al., 2010; Thomas & Lincoln, 2008). Although a greater understanding of psychological stress in individuals with aphasia is beginning to emerge, little remains known about whether the linguistic struggles related to aphasia are associated with a physiological stress response. As previously described by Laures et al. (2007), psychological stress can activate the hypothalamus-pituitary-adrenal (HPA) axis, a neuroendocrine stress response system (Kirschbaum, Pirke, & Hellhammer, 1993). After a stressor is perceived, the HPA axis is activated by the corticotropin-releasing hormone (CRH), which is secreted from the median emininence of the hypothalamus. This hormone then stimulates the pituitary gland to release adrenocorticotrophic hormone (ACTH), which then signals the adrenal glands to produce and release cortisol (Bishop, 1994; Sapolsky, 2002). Cortisol is a glucocorticoid circulating in the human body. The level of cortisol changes in response to stressful or challenging situations. Variations in cortisol allow for glucose release, which provides additional energy to physically manage stressors.
Only a few studies have measured cortisol in individuals with aphasia in an attempt to explore physiological stress in this population. Laures-Gore, Odell, and Coe (2003) studied males with left hemisphere brain damage and aphasia post-stroke (LH; N = 10) and a healthy neurologically intact control group (NI; N = 10) who participated in a linguistic and nonlinguistic vigilance task. Salivary cortisol levels were measured pre- and post-task between 1400 and 1800 hours. Although neither task was sufficient in invoking a physiologic stress response in the aphasia group, it was found that the aphasia group had significantly higher afternoon baseline levels of salivary cortisol than did the non-brain damaged group (M = .19 µg/dl, NI; M = .26 µg/dl, LH). In an effort to determine the type of stimuli that evokes a stress response as reflected by cortisol reactivity in individuals with aphasia, Laures-Gore et al. (2007) investigated the use of salivary cortisol as a marker of stress in individuals with aphasia following a linguistic stressor. A healthy control group and a left-hemisphere stroke with aphasia experimental group participated in both a linguistic and a nonlinguistic task. The sampling strategy followed the design of the Trier Social Stress Test (Kirschbaum et al., 1993), an established laboratory stress test that evokes strong cortisol reactivity. Salivary cortisol was measured before and following each task between 1330 and 1800 hours. The control group demonstrated cortisol reactivity following the linguistic stressor; however, contrary to expectations, the group with aphasia did not. In fact, their response steadily declined following the linguistic task. In contrast to Laures-Gore et al. (2003), Laures-Gore et al. (2007) did not find differences in afternoon baseline cortisol levels between the healthy control group and the aphasia group (M = .29 µg/dl, NI; M = .3 µg/dl, LH).
The differing results regarding baseline afternoon salivary cortisol levels from the two studies suggest that further exploration is needed to better understand the relation between aphasia and salivary cortisol levels. Additionally, given the potential impact of the neurological changes related to stroke and stroke laterality on salivary cortisol secretion (Christensen, Boysen, & Johannesen, 2004; Fassbender, Schmidt, Mossner, Daffertshofer, & Hennerici, 1994; Feibel, Hardy, Campbell, Goldstein, & Joynt, 1977; Johansson, Ahren, Nasman, Carlstrom, & Olsson, 2000; Lueken et al., 2009; Makikallio et al., 2007; Marklund, Peltonen, Nilsson, & Olsson, 2004; Oka, 1956; Olsson, 1990; Schwarz, Schwab, Klinga, Maser-Gluth, & Bettendorf, 2003; Tchiteya, Lecours, Elie, & Lupien, 2003; Wang et al., 2005; Wittling & Pfluger, 1990; Wittling & Schweiger, 1993), a right hemisphere brain-damaged comparison group with language compromise should be included, in contrast to the previous studies by Laures-Gore and colleagues which used only healthy neurologically intact control groups. Therefore, the current study explores physiological stress in aphasia patients by measuring afternoon salivary cortisol levels over time and relating these levels to aphasia severity, while comparing cortisol levels in a non-aphasic post-stroke comparison group. It is hypothesized that if the linguistic struggles of aphasia create a physiological stress response, salivary cortisol levels will decline as recovery from aphasia occurs. It is predicted that the right hemisphere brain-damaged comparison group will demonstrate lower salivary cortisol levels because they do not have aphasia. Improving our understanding of the relation between aphasia and physiologic stress will eventually inform clinical interventions and advance pharmacological and behavioral therapeutic techniques. Furthermore, exploring cortisol levels over time is important because aphasic patients’ physical and mental health may be negatively affected if cortisol remains elevated for a protracted period (Crofford et al., 2004; Farhadi et al., 2005; Fei, Liu, Zhang, & Zhou, 2004; Mussi et al., 2003).
Method
Participants
Participants with left hemisphere stroke and aphasia (LH) and right hemisphere stroke (RH) were recruited via word-of-mouth through speech-language pathology departments in area hospitals. No participants with a history of multiple strokes or head injury per caretaker or self-report, receiving non-oral nutrition, or taking corticosteroid medications were included. Forty-two individuals initially consented in the study. However, eleven individuals were excluded from data analysis due to the identification of bilateral strokes following receipt of MRI reports (N = 2), inability to complete the entire study due to recurrent stroke (N = 1), development of visual impairment (N = 1), no MRI or CT report available (N = 1), and voluntary withdrawal due to the study’s time commitment or other personal reasons (N = 6). Nineteen LH (8 males, 11 females) and 12 RH (8 males, 4 females) participants were included in this study. Mean age was 55.7 years (SD = 9.1) for LH and 58.5 years (SD = 8.4) for the RH group. No significant age difference was found between groups (t = −.86, p = .77). All participants began the study between 1–6 months post onset of their stroke. Mean initiation of study was 2.4 months post onset (SD = 1.3 months) for LH and 2.4 months post onset (SD = 1.6 months) for the RH group. No significant differences in months post onset were found between groups (t = .11, p = .83). Fifteen LH participants were Caucasian and four were African-American. Six RH participants were Caucasian and six were African-American. Mean years of education for LH was 14.4 (SD = 1.9) and 12.8 (SD = 3.7) for the RH group. No significant differences were found between groups (t = 1.65, p = .17). All individuals passed a hearing screening at .5, 1, and 2 KHz at 45 dB bilaterally. All participants passed a vision screening using a Snellen chart by demonstrating at least 20/40 corrected vision. See Tables 1 and 2 for participant descriptions. A subset of these participants were also described in Laures-Gore, Marshall, and Verner (2011).
Table 1.
Description of LH Participants
| Subject | Gender | Age | Ethnicity | Site of Stroke | MPO | WAB AQ |
Initial Aphasia type |
|---|---|---|---|---|---|---|---|
| 1 | F | 60.3 | C | L MCA | 1.00 | 85.5 | Anomic |
| 2 | M | 70.1 | C | L MCA | 1.25 | 14.1 | Broca’s |
| 3 | F | 55.7 | AA | L Parietal | 1.50 | 77.3 | Anomic |
| 4 | M | 41.9 | C | L MCA | 1.25 | 63.5 | Transcortical Sensory |
| 5 | F | 52.3 | C | L MCA | 1.00 | 34.2 | Broca’s |
| 6 | F | 52.3 | C | L MCA/ACA | 2.00 | 17.6 | Broca’s |
| 7 | F | 40.8 | C | L MCA | 0.50 | 88.6 | Anomic |
| 8 | F | 55.5 | C | L MCA | 2.25 | 89.6 | Anomic |
| 9 | F | 47.3 | AA | L MCA with thrombus | 1.25 | 82.6 | Anomic |
| 10 | M | 62.5 | C | L MCA | 2.25 | 68.5 | Transcortical Motor |
| 11 | M | 53.5 | AA | L Anterior, Temporal, Mid-Parietal | 3.50 | 13.2 | Global |
| 12 | F | 74.3 | C | L MCA | 4.50 | 42.1 | Broca’s |
| 13 | F | 62.8 | C | L Basal Ganglia | 5.00 | 64.6 | Broca’s |
| 14 | F | 46.1 | C | L ICA with aneurysm | 2.00 | 84.8 | Anomic |
| 15 | M | 51.0 | C | L MCA/PCA | 2.00 | 19.5 | Broca’s |
| 16 | F | 55.7 | C | L MCA | 3.25 | 36.9 | Broca’s |
| 17 | M | 66.3 | C | L ICA | 3.50 | 87.6 | Anomic |
| 18 | M | 61.7 | C | L ICA | 4.75 | 93.0 | Anomic |
| 19 | M | 48.8 | AA | L MCA/ICA | 3.50 | 35.8 | Broca’s |
Note. MCA = Middle Cerebral Artery; ICA = Internal Carotid Artery; PCA = Posterior Cerebral Artery; MPO= months post onset; WAB= Western Aphasia Battery; MIRBI= Mini Inventory of Right Brain Injury; M/F= Male/Female; C/AA= Caucasian/African-American.
Table 2.
Description of RH Participants
| Subject | Gender | Age | Ethnicity | Site of Stroke | MPO | MIRBI |
|---|---|---|---|---|---|---|
| 1 | M | 66.5 | C | R frontal, occipital | 1.50 | 4 |
| 2 | M | 47.8 | AA | R watershed MCA | 1.75 | 4 |
| 3 | F | 61.7 | AA | R MCA/ACA | 1.75 | 6 |
| 4 | F | 57.2 | AA | R MCA/ACA | 2.25 | 6 |
| 5 | F | 61.7 | AA | R MCA | 1.75 | 7 |
| 6 | M | 59.5 | AA | R MCA/PCA | 1.25 | 4 |
| 7 | M | 63.1 | C | R MCA/ACA | 1.25 | 6 |
| 8 | M | 54.3 | C | R basal ganglia | 2.25 | 5 |
| 9 | M | 43.8 | C | R pontine | 4.50 | 7 |
| 10 | F | 50 | AA | R MCA | 6.75 | 6 |
| 11 | M | 63 | C | R MCA | 1.50 | 7 |
| 12 | F | 73.8 | C | R frontal, parietal, temporal | 2.00 | 6 |
Note. MCA = Middle Cerebral Artery; ACA = Anterior Cerebral Artery; PCA = Posterior Cerebral Artery; MPO= months post onset; WAB= Western Aphasia Battery; MIRBI= Mini Inventory of Right Brain Injury; M/F= Male/Female; C/AA= Caucasian/African-American.
Procedure
Language testing
Both groups participated in language testing monthly at their home or at the Georgia State University Speech and Hearing Clinic. All LH participants were given the Western Aphasia Battery (WAB; Kertesz, 1982) and the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001) four times over three months (0, 4, 8, 12 weeks post enrollment in the study). The WAB Aphasia Quotient (AQ) is a composite score indicating aphasia severity, and the BNT provides a raw score for determination of naming accuracy (Kaplan et al, 2001; Kertesz, 1982). Mean time between administration of testing was 30 days (SD = 3.23 days).
Individuals from the RH group were administered the BNT and Mini-Inventory of Right Brain Injury-2 (MIRBI-2; Pimenthal & Knight, 2000) a total of four times over the course of three months. The MIRBI-2 assesses cognitively-based linguistic problems (e.g., metaphor interpretation; visuo-spatial, writing, and reading skills) and provides an overall stanine score reflecting severity of impairment. Mean time between administration of testing was 29.8 days (SD = 4.32 days). Additionally, the RH group was administered the WAB during the initial testing session to rule out presence of aphasia. No RH participants included in the study demonstrated aphasia as defined by a WAB AQ score lower than 93.8; however, they did demonstrate mild to moderate cognitively-based linguistic deficits as indicated by their MIRBI scores.
Salivary cortisol sampling
All participants were seen for saliva sampling biweekly for three months for a total of six samples (0, 2, 4, 6, 8, 10 weeks following enrollment in study). As there were occasional scheduling conflicts, the mean time between samples across all participants was 14.8 days (SD = 4.37). Participants were seen between 1600 and 1800 hours (M = 1637 hours) either in their home or in the Communication Sciences and Disorders Laboratory at Georgia State University. One sample per day was chosen to decrease sampling burden on the participant (Ice et al., 2004). Afternoon measurement was chosen as it tends to be a more stable time period for diurnal variation of salivary cortisol and has been used previously for measurement of stress in LH participants (Anders, 1982; Laures-Gore et al., 2003; Laures-Gore et al., 2007). All participants were instructed to not ingest caffeine, food, nicotine, or alcohol two hours prior to cortisol sampling. Trained graduate assistants obtained the saliva samples. Following a ten minute relaxation period (participants were instructed to sit quietly and relax), each participant chewed on a Salivette (Sarstedt, Rommelsdorf, Germany) for one minute until saturation of the Salivette occurred. All participants were able to orally manipulate the Salivette safely. Samples were frozen at −80 degrees C and stored on the campus of Georgia State University. They were later analyzed by immunoassay using a commercially-prepared kit produced by Diagnostics Systems Laboratory (Webster, TX) at Yerkes Primate Research Center, Emory University, Endocrine Core Laboratory, Atlanta, Georgia.
Results
The WAB Aphasia Quotient (AQ) was used as a dependent measure to indicate change over time within the LH group as it provides an overall severity rating for aphasia (Shewan & Kertesz, 1984). The MIRBI composite score was used to evaluate change in cognitively-based language disorder over time in the RH group. Raw scores from the BNT were used to measure change in naming skills over time in both groups. Multivariate analysis of variance (MANOVA) was used to examine group and time-point differences in cortisol and BNT test results over the course of 3 months (repeated measures). A one-way repeated measures ANOVA was used to examine changes in WAB AQ scores in the LH group and MIRBI scores in the RH group. Pearson’s correlations were performed to evaluate the association between the averaged salivary cortisol levels and averaged language scores within groups. Analysis was completed using SPSS (v. 17.0, 2009) with alpha set at .05.
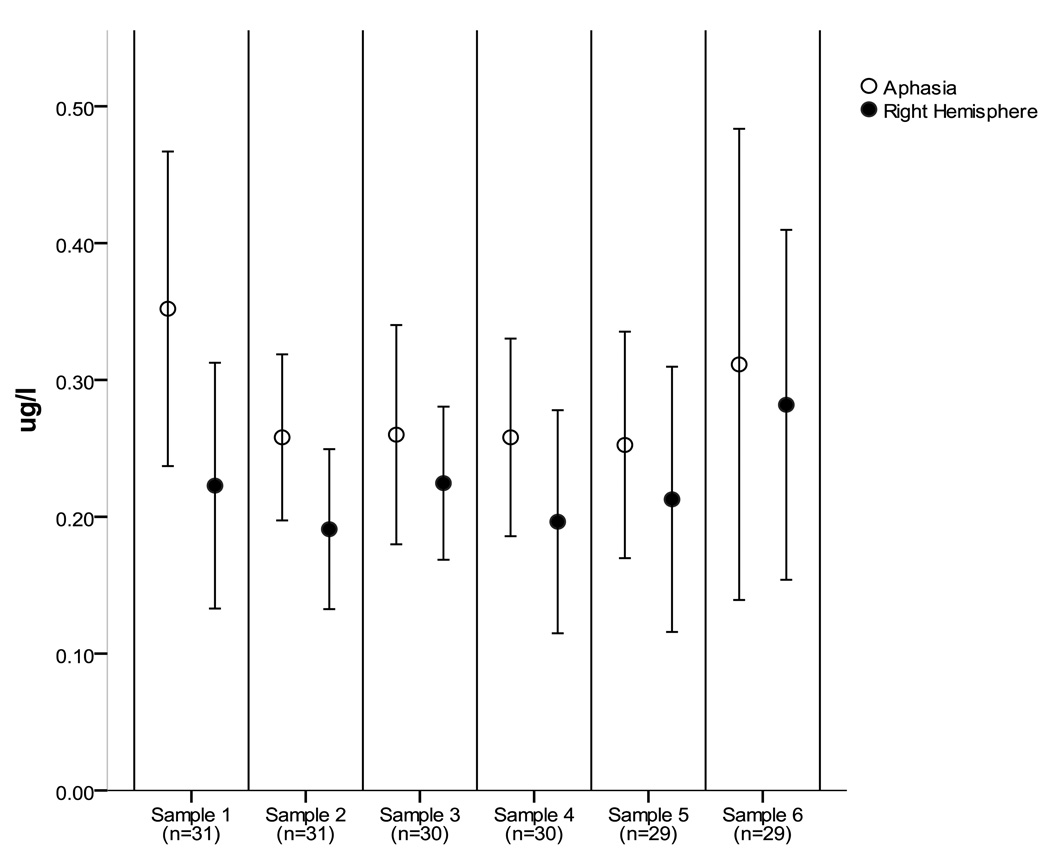
See Tables 3 and 4 for mean test scores for each group over time. See Figure 1 for mean salivary cortisol levels for each group over time. Cortisol sample six from 17 LH was discarded as an outlier due to the value being greater than two standard deviations above the mean. Significant differences between groups on the BNT [F(1,25) = 6.58, p < .017, η2 = .21], changes in BNT scores over time [F(3,23) = 7.68, p < .001, η2 = .24], and marginally significant differences in changes between groups in BNT scores over time [F(3,23) = 2.51, p = .066, η2 = .09] were found. Within the LH group, a significant change over time in the WAB AQ score [F (3, 15) = 16.5, p < .001, η2 = .50] was found. Within the RH group, significant changes in MIRBI scores over time [F(3,8) = 4.04, p = .016, η2 = .288] were found. No significant differences between groups in cortisol [F(1,26) = 1.64, p = .21, η2 = .06], no changes in cortisol over time [F(5,22) = .83, p = .53, η2 = .03], and no significant differences in changes in cortisol over time between groups [F(5,22) = .32, p = .09, η2 = .01] were found.
Table 3.
Test Scores Over Time for Individuals with Aphasia
|
Measure |
Time 1 Baseline |
Time 2 4 weeks |
Time 3 8 weeks |
Time 4 12 weeks |
|---|---|---|---|---|
| WAB-AQ | 57.8 (29.3) | 63.8 (31.4) | 64.7 (32.2) | 72.6 (27.9) |
| BNT | 29.0 (22.0) | 36.37 (22.9) | 36.0 (22.7) | 38.6 (22.1) |
Note. Time points present time from enrollment in study, participants averaged approximately 2.4 months post-stroke before enrollment.
Table 4.
Test Scores Over Time for Individuals with Right Hemisphere Stroke
|
Measure |
Time 1 Baseline |
Time 2 4 weeks |
Time 3 8 weeks |
Time 4 12 weeks |
|---|---|---|---|---|
| BNT | 52.9 (6.6) | 55.0 (6.0) | 55.8 (4.7) | 54.6 (6.2) |
| MIRBI | 5.7 (1.2) | 6.3 (1.5) | 6.3 (1.4) | 6.3 (1.3) |
Note. Time points present time from enrollment in study, participants averaged approximately 2.4 months post-stroke before enrollment.
Figure 1.
Mean cortisol level by group over time
Pearson’s correlation analyses of the sum of salivary cortisol levels over time and the sum of language scores indicates no significant relation between cortisol and naming skills in the LH group (BNT, r = .25, p = .22) and no relation between overall aphasia severity and salivary cortisol (WAB AQ, r = .26, p = .28). A significant negative correlation was found between salivary cortisol and naming skills in the RH group (BNT, r = −.64, p = .03). No correlation was found between salivary cortisol and severity of cognitively-based language disorders in the RH group (MIRBI, r = −.50, p = .10]. Sum salivary cortisol levels and sum test scores were used because there was not a one-one correspondence between time of salivary cortisol sampling and language testing.
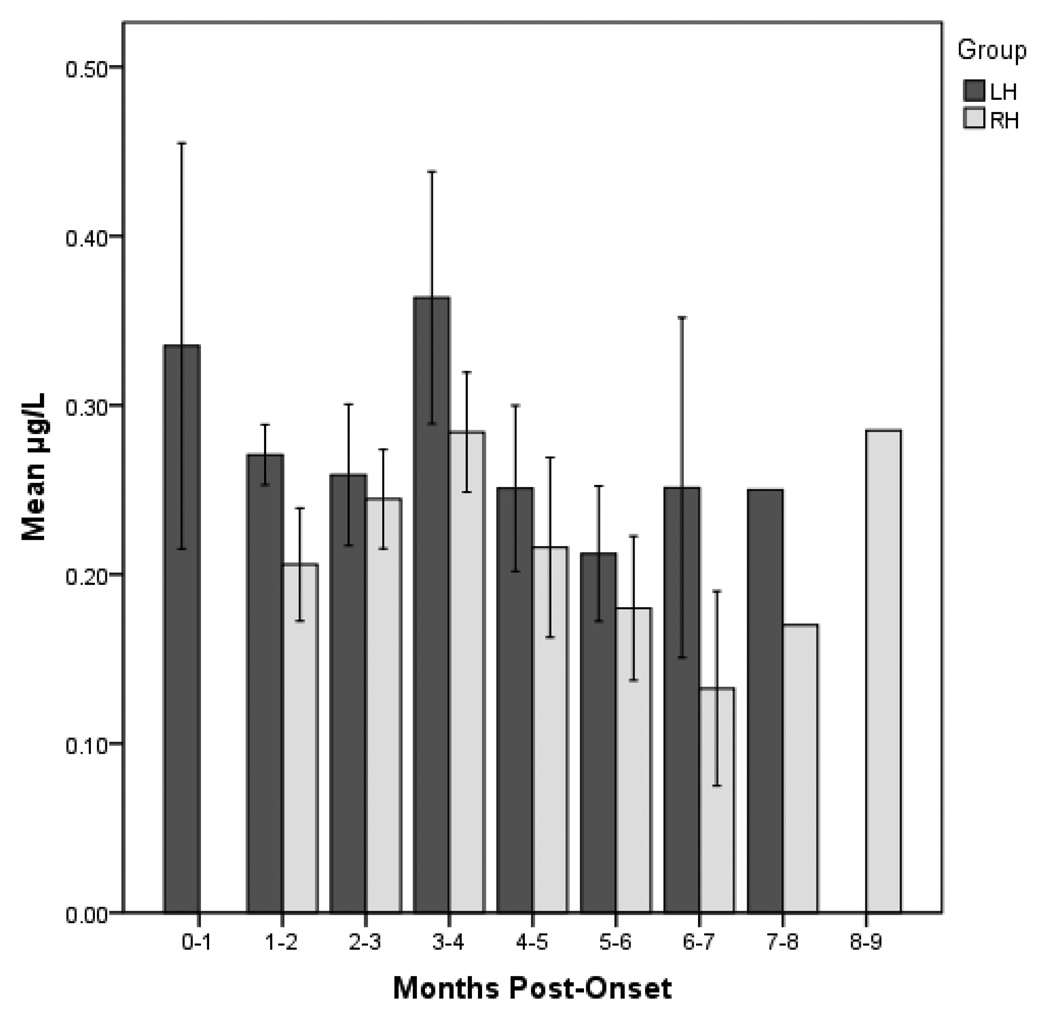
Because the participants initiated the study at different time points post-stroke (1–6 months), a post hoc analysis of salivary cortisol levels was conducted to determine if the lack of significant differences in salivary cortisol are a function of time post onset at baseline. An independent samples t-test was conducted on the median split for each group (LH and RH) for time post-onset at baseline [Mdn LH = 2.03 months, N = 10 (early), N = 9 (late); Mdn RH = 1.76 months, N = 6 (early), N = 6 (late)]. Results indicate a marginally significant difference between early and late baseline levels within the LH group (t = 1.897, p = .08; Early M = .26 ug/dl, SD = .13 Late M = .44 ug/dl, SD = .27). The effect size of this difference was large (d = .86), despite the failure to reach statistical significance. No significant difference was found between early and late baseline levels within the RH group (t = −.230, p = .82, d = .13; Early M = .22 ug/dl, SD = .09; Late M = .24 ug/dl, SD = .07). Additionally, cortisol levels were grouped according to time post onset (See Figure 2). Descriptive analyses indicate trends toward the LH group demonstrating higher baseline salivary cortisol levels than the RH group at all baseline time points.
Figure 2.
Cortisol levels categorized by months post-onset
Discussion
The current study explored physiological stress in aphasia patients by relating afternoon salivary cortisol levels over time to aphasia severity, while comparing cortisol levels in a non-aphasic post-stroke group. It was predicted that salivary cortisol levels would decline as recovery from aphasia occurred and that a right hemisphere brain-damaged comparison group would demonstrate lower salivary cortisol levels over time because they do not have aphasia. The results indicate that although both groups demonstrate linguistic improvements, neither group exhibited changes in salivary cortisol levels over time. Interestingly, there was a negative relation between naming skills in the right hemisphere group, suggesting that better naming performance results in lower afternoon salivary cortisol levels. Although the LH group consistently demonstrated descriptively higher levels of afternoon salivary cortisol levels than the RH across all time points, no significant difference between the groups exists.
The findings suggest that stroke patients with aphasia do not exhibit higher levels of afternoon salivary cortisol levels than another stroke group with a communication disorder. Over the years, there have been numerous reports of stress-related reactions in individuals with aphasia, such as anxiety, depression, frustration, and social isolation (e.g., Gainotti, 1997; Sapir & Aronson, 1990; Starkstein & Robinson, 1988). The current finding that cortisol levels are unrelated to changes in aphasia severity was not predicted given these clinical observations and recent reports of psychological stress in individuals with aphasia (Hilari et al., 2010; Laures-Gore et al., 2007a; Thomas & Lincoln, 2008). Individuals with right hemisphere damage are often described as having limited awareness of their stroke-related deficits (Jehkonen, Laihosalo, & Kettunen, 2006; Kortte & Hillis, 2009). Therefore, the lack of heightened cortisol levels associated with post-stroke behavioral change in the RH group is less surprising, as one might predict that they would perceive fewer deficits and less stress related to their change in cognitive-linguistic skills. However, it is surprising that cortisol levels did not differ between the two groups given evidence suggesting right hemispheric control of cortisol secretion (Wang et al., 2005; Wittling & Pfluger, 1990). Additionally, the current finding contrasts with Lueken et al. (2009) who found higher levels of cortisol in left hemisphere stroke patients than healthy controls or right hemisphere stroke patients. This contradiction could be related to differences in the studies regarding time post onset of stroke and cortisol sampling strategy (morning vs. afternoon collection of saliva samples).
The observed qualitatively higher afternoon salivary cortisol levels in the aphasia group should be highlighted. The current data reveal a rather large standard deviation in cortisol levels for both groups, suggesting significant individual differences on this factor that may be obscuring lateral differences in this sample. The lack of observed significant differences between the groups in regards to salivary cortisol levels may be more a function of sample size rather than effect size. A follow-up study with a larger sample size is recommended.
In the current study, one should consider the salivary cortisol sampling strategy. The diurnal variation of cortisol in healthy individuals is one that follows a typical pattern of higher levels in the morning with a rapid decline that slows by the afternoon (Anders, 1982). Therefore, afternoon sampling was chosen based on this cortisol stability. However, there is evidence that there is more heterogeneity in the diurnal variation of cortisol than previously believed. Altered cortisol diurnal patterns have been associated with pathophysiologic states related to pain and fatigue, such as rheumatoid arthritis, fibromyalgia, and cancer (Bower et al, 2004; Crofford et al., 1994; Neeck, Federlin, Graef, Rusch, & Schmidt, 1990), or chronic stress related to unemployment (Ockenfels et al., 1995). Although it is thought that cortisol secretion is altered acutely following stroke with a typical profile of increased secretion (Feibel et al., 1977; Olsson, 1990), little is known about the diurnal variation of cortisol following stroke (Franceschini et al., 1994; Tchiteya et al., 2003). It may be that the sampling strategy should not occur in the afternoon, but instead during the morning hours. However, the stability of the afternoon measures of cortisol over the course of three months for both stroke groups should be emphasized. This appears to be the first evidence that afternoon cortisol levels are maintained over time after stroke. Two previous studies examining diurnal variation of cortisol followed the patients for either 24 hours or 5 days post-stroke (Franceschini et al., 1994; Tchiteya et al., 2003). Future studies should address the diurnal cycle of stress biomarkers in left and right hemisphere stroke patients across recovery in order to better understand the interaction of language disorders and physiological stress.
Overall, the current findings suggest that afternoon salivary cortisol levels do not change as recovery from aphasia occurs. Salivary cortisol levels are associated with naming skills, but not overall severity of language impairment, in right hemisphere damaged patients. Because the complex interplay of stroke, aphasia, and the subsequent physiological stress response is just beginning to be explored in humans, further study of this area is warranted to more fully understand this interaction.
Acknowledgments
This study was supported by NIH R03 DC006177 awarded to the author. The author would like to thank the following speech-language pathologists: Winn Hill, Scott Russell, Julie Bonner, Michelle Cooper, Amy Dryver, Amy Samuelson, Cheryl Stewart, and Tracey Wallace. Additional gratitude is extended to the participants. I am appreciative of Robin Morris, Christine Heim, Bryan Williams and Tony Buchanan for their helpful editorial comments and Lauren Clepper and Michaela DuBay for their assistance with data preparation.
References
- Anders TF. Biological rhythms in development. Psychosomatic Medicine. 1982;44:61–72. doi: 10.1097/00006842-198203000-00008. [DOI] [PubMed] [Google Scholar]
- Bishop GD. Health psychology. Boston: Allyn & Bacon; 1994. [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Christensen H, Boysen G, Johannesen HH. Serum-cortisol reflects severity and mortality in acute stroke. Journal of the Neurological Sciences. 2004;217:175–180. doi: 10.1016/j.jns.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, Wilder RL. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromayalgia. Arthritis & Rheumatism. 1994;37:1583–1592. doi: 10.1002/art.1780371105. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Young EA, Engleber NC, Korszun A, Brucksch CB, McClure LA, Demitrack MA. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain, Behavior, and Immunity. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Keshavarzian A, Van de Kar LD, Jakate S, Domm A, Zhang L, Fields JZ. Heightened responses to stressors in patients with inflammatory bowel disease. American Journal of Gastroenterology. 2005;100:1796–1804. doi: 10.1111/j.1572-0241.2005.50071.x. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Schmidt R, Mossner R, Daffertshofer M, Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke. 1994;25:1105–1108. doi: 10.1161/01.str.25.6.1105. [DOI] [PubMed] [Google Scholar]
- Fei GH, Liu RY, Zhang ZH, Zhou JN. Alterations in circadian rhythms of melatonin and cortisol in patients with bronchial asthma. Acta Pharmacologia. 2004;25:651–656. [PubMed] [Google Scholar]
- Feibel J, Hardy P, Campbell R, Goldstein M, Joynt R. Prognostic value of the stress response following stroke. Journal of the American Medical Association. 1977;238:1374–1376. [PubMed] [Google Scholar]
- Franceshini R, Gandolfo C, Cataldi A, Del Sette M, Cianciosi P, Finocchi C, Barreca T. Twenty-four-hour beta-endorphin secretory pattern in stroke patients. Stroke. 1994;25:2142–2145. doi: 10.1161/01.str.25.11.2142. [DOI] [PubMed] [Google Scholar]
- Hilari K, Northcott S, Roy P, Marshall J, Wiggins RD, Chataway J, Ames D. Psychological distress after stroke and aphasia: the first six months. Clinical Rehabilitation. 2010;24(2):181–190. doi: 10.1177/0269215509346090. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Laihosalo M, Kettunen J. Impact of neglect on functional outcome after stroke - a review of methodological issues and recent research findings. Restorative Neurology and Neuroscience. 2006;24:209–215. [PubMed] [Google Scholar]
- Johansson A, Ahren B, Nasman B, Carlstrom K, Olsson T. Cortisol axis abnormalities early after stroke-relationships to cytokines and leptin. Journal of Internal Medicine. 2000;247:179–187. doi: 10.1046/j.1365-2796.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea & Febiger; 2001. [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The "Trier Social Stress Test": A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kortte K, Hillis AE. Recent advances in the understanding of neglect and anosognosia following right hemisphere stroke. Current Neurology & Neuroscience Reports. 2009;9:459–465. doi: 10.1007/s11910-009-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laures-Gore J, Hamilton A, Matheny K. Coping resources, perceived stress, and life experiences in individuals with aphasia. Journal of Medical Speech-Language Pathology. 2007a;15:423–431. [Google Scholar]
- Laures-Gore J, Heim C, Hsu YS. Assessing cortisol reactivity to a linguistic task as a marker of stress in individuals with left hemisphere stroke and aphasia. Journal of Speech-Language-Hearing Research. 2007b;50:493–507. doi: 10.1044/1092-4388(2007/034). [DOI] [PubMed] [Google Scholar]
- Laures JS, Odell KH, Coe C. Arousal and auditory vigilance in individuals with aphasia during a linguistic and nonlinguistic vigilance task. Aphasiology. 2003;17:1133–1152. [Google Scholar]
- Laures-Gore JS, Marshall RS, Verner E. Digit span differences in aphasia and right brain damage. Aphasiology. 2011;25(1):43–56. doi: 10.1080/02687031003714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Leisse M, Mattes K, Naumann D, Wittling W, Schweiger E. Altered tonic and phasic cortisol secretion following unilateral stroke. Psychoneuroendocrinology. 2009;34:402–412. doi: 10.1016/j.psyneuen.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Marklund N, Peltonen T, Nilsson T, Olsson T. Low and high circulating cortisol levels predict mortality and cognitive dysfunction early after stroke. Journal of Internal Medicine. 2004;256:15–21. doi: 10.1111/j.1365-2796.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- Mussi C, Angelini C, Crippa S, Caprotti R, Fumagalli L, Motta V, Uggeri F. Alteration of hypothalamus-pituitary-adrenal glands axis in colorectal cancer patients. Hepatogastroenterology. 2003;50:228. [PubMed] [Google Scholar]
- Neeck G, Federlin K, Graef D, Rusch D, Schmidt K. Adrenal secretion of cortisol in patients with rheumatoid arthritis. Journal of Rheumatology. 1990;17:24–29. [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: Overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Oka M. Effect of cerebral vascular accident on the level of 17-hydroxycorticosteroids in plasma. Acta Med Scandinavia. 1956;156:221–226. doi: 10.1111/j.0954-6820.1956.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Olsson T. Urinary free cortisol excretion shortly after ischaemic stroke. Journal of Internal Medicine. 1990;228:177–181. doi: 10.1111/j.1365-2796.1990.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Pimenthal P, Knight J. Mini-Inventory of Right Brain Injury-2. Austin, Tx: Pro-Ed; 2000. [Google Scholar]
- Sapolsky R. Endocrinology of the stress response. In: Becker J, Breedlove S, Crews D, McCarthy M, editors. Behavioral endocrinology. Cambridge, MA: MIT Press; 2002. pp. 409–450. [Google Scholar]
- Shewan CM, Kertesz A. Effects of speech and language treatment on recovery from aphasia. Brain and Language. 1984;23:272–299. doi: 10.1016/0093-934x(84)90068-3. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Schwab S, Klinga K, Maser-Gluth C, Bettendorf M. Neuroendocrine changes in patients with acute space occupying ischaemic stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:725–727. doi: 10.1136/jnnp.74.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchiteya B, Lecours A, Elie R, Lupien S. Impact of unilateral brain lesion on cortisol secretion and emotional state: Anterior/posterior dissociation in humans. Psychoneuroendocrinology. 2003;28:674–686. doi: 10.1016/s0306-4530(02)00050-1. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Lincoln NB. Predictors of emotional distress after stroke. Stroke. 2008;39:1240–1245. doi: 10.1161/STROKEAHA.107.498279. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittling W, Pflueger M. Neuroendocrine hemisphere asymmetries: Salivary cortisol secretion during lateralized viewing of emotion-related and neutral films. Brain and Cognition. 1990;14:243–265. doi: 10.1016/0278-2626(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Wittling W, Schweiger E. Neuroendocrine brain asymmetry and physical complaints. Neuropsychologia. 1993;31(6):591–608. doi: 10.1016/0028-3932(93)90054-4. [DOI] [PubMed] [Google Scholar]