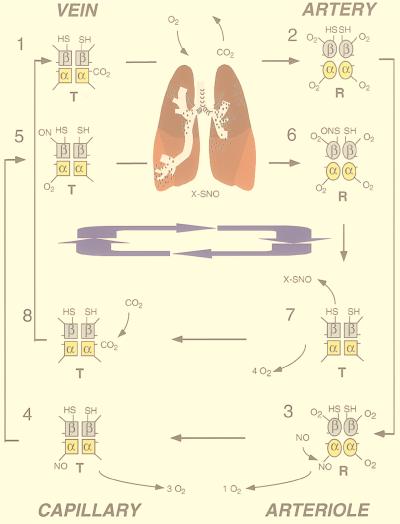
Figure 1.
This model depicts the physiologically relevant reactions of NO with Hb that have been proposed to occur during the respiratory cycle in erythrocytes (1, 3, 16, 17). Notably, these are the reactions that are considered to be most relevant for controlling NO bioactivity in blood vessels. An important aspect is that these reactions are modulated by R to T state structural transitions of Hb, assuming the simple two-state paradigm (49). Stages of addition and release of NO, O2, and CO2 by a single molecule of Hb are shown as Hb makes two complete cycles through the circulation. The first cycle is represented by the series of Hb molecules labeled 1–4, and the second cycle by molecules labeled 5–8. Molecule 8 is ready to begin the cycle anew as molecule 1. Essential features are the efficient capture of NO by Fe(II)-Hb in T structure (molecule 3), formation of SNO-Hb by NO transfer from Fe(II) to βCys93 (molecule 6), and facilitated transnitrosation to produce X-SNO upon transition of Hb from R to T structure (molecule 8). Over the two cycles, there is a net scavenging of one molecule of NO that is subsequently released as a bioactive NO equivalent (X-SNO, which can depart from the erythrocyte and elicit vascular responses). The Hb molecule shown represents approximately 1 in 1,000 that may carry NO at any given moment (3).