Figure 1.
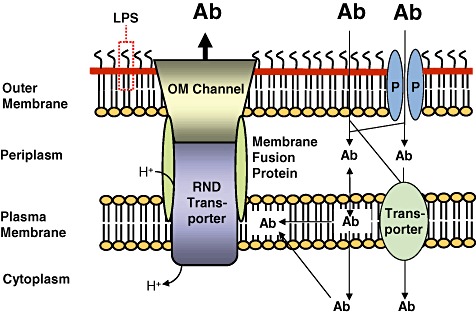
The cell envelope of Gram‐negative bacteria is a major barrier for antibiotics. The cell envelope of Gram‐negative bacteria consists of the plasma membrane, the periplasm and the outer membrane. The outer membrane is the major barrier for antibiotics (Ab). Some antibiotics penetrate this membrane either through porins (P) or by passive diffusion through the outer membrane phospholipid (inner leaflet)‐lipid A (outer leaflet) bilayer. The lipopolysaccharide (LPS) forms another barrier for many antibiotics but polycationic compounds such as gentamicin and colistin are being transported through the outer membrane via interaction with LPS in a process called self‐promoted uptake. Antibiotic molecules then enter the cell from the periplasm either via partition into and passive diffusion through the plasma membrane or are actively transported via transporters into the cytoplasm. Efflux pumps of the resistance nodulation cell division (RND) superfamily are major players in antibiotic resistance of Gram‐negative bacteria. These tripartite systems span the entire cell envelope and are composed of an RND transporter, a membrane fusion protein and an outer membrane (OM) channel. It is generally accepted that RND transporters acquire substrates from the plasma membrane. Efflux via RND pumps is driven by the proton gradient. The setup illustrated in this figure explains why synergy between exclusionary outer membrane and/or cell envelope properties is a powerful mechanism leading to high‐level antibiotic resistance in non‐enteric Gram‐negative bacteria. Although antibiotics may be present outside the bacterial cell in high concentration (illustrated by large bold letters), passive influx through the various compartments of the cell coupled to active efflux via a cell envelope‐spanning efflux system results in low intracellular concentrations of antibiotics (illustrated by smaller letters).
