Fig. 1.
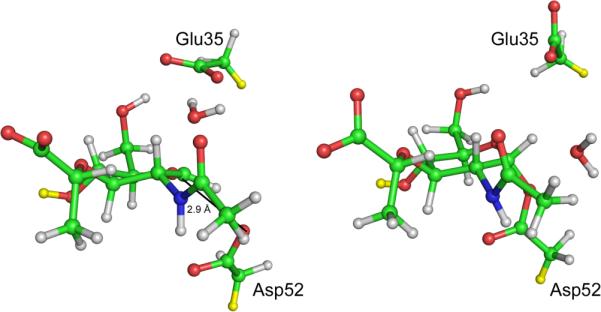
Lysozyme is an example of a classic `textbook' enzyme, and was the first to have its mechanism proposed based on structural data. However, the previously commonly taught mechanism (where the reaction proceeds via a oxocarbenium ion) is probably wrong. Crystallography and electrospray ionization mass spectrometry of mutant hen egg white lysozyme, or a fluorinated substrate, support formation of a covalent intermediate. QM/MM calculations also support formation of the covalent intermediate in the wild type enzyme with the natural substrate.3 Representative snapshots from QM/MM (PM3/CHARMM22) umbrella sampling molecular dynamics simulations of the transition state (left hand side) and the covalent intermediate (right hand side) are shown. Only atoms in the QM region are shown, for clarity (i.e. the D site NAM sugar and the side-chains of Glu35 and Asp52). The distance between Asp52 Od2 and the D site NAM C1 decreases from ~ 2.9 Å in transition state (indicated in the figure) to ~ 1.4 Å in the covalent intermediate. `Link' atoms are shown in yellow: these are QM hydrogen atoms added to the system at the QM/MM boundary to satisfy the bonding requirement of QM atoms where the boundary separates covalently bonded atoms. Reproduced from ref.3
