FIGURE 3. Various ER chaperones are released from thapsigargin-treated fibroblasts and thapsigargin-induced surface calreticulin expression is independent of calreticulin-ERp57 binding.
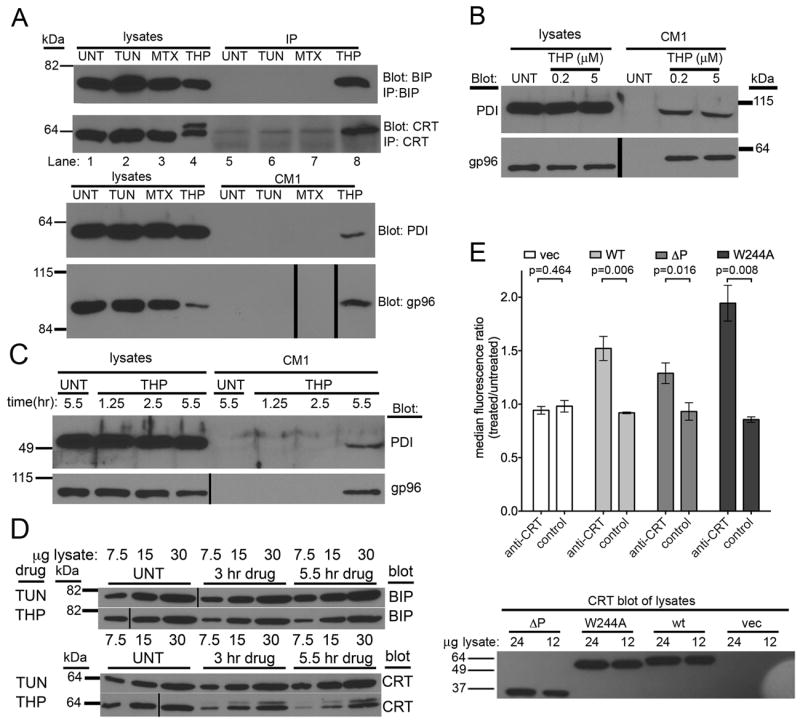
(A) Immunoblotting of cell supernatants and lysates for the presence of various ER chaperones. Supernatants (CM1) from cells treated with TUN, MTX or THP or untreated cells were harvested in parallel with the corresponding cell lysates. Direct immunoblotting analyses of each fraction were performed for PDI and gp96. For CRT and BiP, proteins in cell supernatants were first immunoprecipitated (IP) with anti-CRT or anti-BiP, prior to immunoblotting analyses. (B) Comparison of PDI & gp96 release in response to low or high thapsigargin dose (200 nM or 5 μM). (C) Kinetics of PDI and gp96 secretion. Analyses were performed as in A, but immunoblotting analyses were undertaken at the indicated time points. (D) Kinetics and magnitude of BiP and CRT induction in response to TUN & THP. Indicated microgram amounts of total cell lysates were analyzed as in A at the indicated time-points post-drug treatments. (E) Flow cytometric assessments of ERp57-binding dependence of calreticulin surface expression. Cell-surface calreticulin measured as in Figure 1 before and after thapsigargin (5-7 hrs of 5 μM THP) treatment of CRT-/- MEFs infected with retroviruses encoding wild calreticulin (WT), a calreticulin construct lacking the P-domain (ΔP), a calreticulin point mutant that is unable to interact with ERp57 (W244A), or a virus lacking calreticulin (vec). Representative blots of 3-4 experiments are shown in A. Data are based on (B and C) 1 experiment, (D) 1 experiment shown, representative of 2 total experiments (E) 3-5 experiments. (A) Approximately 8500 cells per lane contributed to the CM1 used to blot for PDI and gp96. The blots of corresponding lysates show approximately 30,000 cells worth of lysate. (A-D) Vertical black lines in blots indicate places where lanes of the blot were re-arranged to match the labeling scheme used in adjacent immunoblots or to digitally remove lanes containing size standard (relevant bands from size standard are shown digitally for each blot). The p-values from two-tailed, paired t-tests are indicated.
