Abstract
Background:
Atorvastatin has a longer duration of action than other hydroxymethylglutaryl coenzyme A reductase inhibitors.
Objectives:
The objective was to evaluate the efficacy of alternate day vs. daily dosing of atorvastatin for the treatment of hyperlipidemia.
Materials and Methods:
In this prospective, open label, crossover study, 40 patients with plasma low-density cholesterol (LDL-C) of more than 130 mg/dl and total cholesterol (TC) more than 200 mg/dl were recruited. After baseline tests, they were randomly allocated to two groups. Group A received 20 mg atorvastatin on alternate days and group B received 20 mg atorvastatin daily for 12 weeks. After 4 weeks of washout period, the groups were crossed over to the other treatment regimen for another 12 weeks. Fasting plasma lipid profile and serum alanine transaminase (ALT) and aspartate transaminase (AST) were measured for both groups at 6th, 12th, 16th, 22nd, and 28th weeks. Results were pooled across the periods and data between the two groups were compared using unpaired t-test.
Results:
Among the 40 enrolled subjects, 38 completed the study. Both treatment regimens significantly reduced LDL-C and TC compared to baseline. There was no statistically significant difference between the two groups in terms of reduction of plasma LDL-C and TC at 6 and 12 weeks of treatment. Both the regimens were well tolerated.
Conclusion:
Alternate-day treatment with atorvastatin is comparable in efficacy and safety to the established daily treatment regimen, thus being a cost effective alternative.
KEY WORDS: Atorvastatin, hyperlipidemia, alternative dosing regimen
Introduction
Cardiovascular disease (CVD) is one of the leading causes of death in India.[1] The deaths due to CVD in India are more than 25% of all causes of mortality at present and are expected to contribute to more than half the cases of heart disease in the world within the next 15 years.[2] Atherosclerosis of coronary vessels is the main pathognomonic mechanism responsible for CVD and efforts to reduce this provide an important therapeutic strategy to reduce mortality related to acute cardiovascular events. Elevated plasma cholesterol levels have been shown to be a major modifiable risk factor for atherosclerosis and thus presents an important point for intervention in primary prevention of CVD.[3] Hydroxymethylglutaryl coenzyme A (HMGCo A) reductase inhibitors (statins) are well established as treatment for lowering low-density lipoprotein cholesterol (LDL-C) and reducing cardiovascular events.[4–6] Overall, these agents have a remarkable safety profile.[6,7] Atorvastatin, besides being among the most prescribed statins, has a longer effect half-life of 20–30 hours due to active metabolites.[8] In India, therapy with atorvastatin in WHO-recommended dose[9] may cost between [ ] 150.00 and [
] 150.00 and [ ] 450.00 per month (approximately US$ 3.00 to 9.00), and as for several other drug classes, is subject to variation.[10–12] The lower and middle income groups of Indian society are rapidly becoming major sufferers of CVD, causing loss of one quarter of DALYs due to all noncommunicable diseases[13] and the economic burden of atorvastatin therapy may be substantial for this large section of population. Atorvastatin being a drug with long half-life, this study proposes that by using alternate-day doses of atorvastatin a significant LDL-C reduction may still be achieved, while reducing the total cost of treatment. Studies evaluating the effect and tolerance of alternative statin dosing are limited. A limited number of trials have demonstrated efficacy with alternate-day statin dosing including a few anecdotal case reports,[14–17] but till date no such trials have been conducted in India. The study reported here examines the effect and tolerance of every other day atorvastatin therapy in comparison to the established daily statin regimen in reduction of plasma LDL-C.
] 450.00 per month (approximately US$ 3.00 to 9.00), and as for several other drug classes, is subject to variation.[10–12] The lower and middle income groups of Indian society are rapidly becoming major sufferers of CVD, causing loss of one quarter of DALYs due to all noncommunicable diseases[13] and the economic burden of atorvastatin therapy may be substantial for this large section of population. Atorvastatin being a drug with long half-life, this study proposes that by using alternate-day doses of atorvastatin a significant LDL-C reduction may still be achieved, while reducing the total cost of treatment. Studies evaluating the effect and tolerance of alternative statin dosing are limited. A limited number of trials have demonstrated efficacy with alternate-day statin dosing including a few anecdotal case reports,[14–17] but till date no such trials have been conducted in India. The study reported here examines the effect and tolerance of every other day atorvastatin therapy in comparison to the established daily statin regimen in reduction of plasma LDL-C.
Materials and Methods
Prior approval of Institutional Ethics Committee was obtained before commencing the study. The sample size was calculated using the software WinPepi PAIRSetc Version 2.60.
Forty participants of either sex, all aged 18 years and above, were recruited for this prospective, open label, dual arm, crossover study.
The subjects were considered to be eligible for participation if they were diagnosed with hyperlipidemia, meeting the criteria for pharmacological treatment according to National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines.[18]
The exclusion criteria included significant hypertriglyceridemia (>400 mg/dl); abnormal serum ALT, AST; concurrent administration of immunosuppressants, azole antifungal agents, protease inhibitors; hypothyroidism; pregnancy/lactation; history of statin use within last 3 months or concurrent cholesterol lowering medication; prior sensitivity/intolerance to any HMG CoA reductase inhibitor; and patient with reported nonadherence to any lipid-altering agent in past.
Prior to participation to the study, voluntary informed consent was obtained from all participants in a prescribed format.
Baseline investigations for all subjects consisted of complete medical history with concurrent medication use, thorough physical examination including recording of height, weight, blood pressure and other vital signs; and laboratory reports including fasting (12 hours) plasma lipid profile, liver function tests with serum ALT and AST estimation, complete hemogram, fasting blood glucose, plasma urea and creatinine; 12 lead ECG and chest X RAY PA view.
All subjects were advised appropriate diet regimens based on NCEP step II diet. They were counseled about the probable adverse effects of atorvastatin including those on the hepatic and musculoskeletal system; and asked to contact the investigator in case they experienced any muscle pain/cramps, malaise, pale stool or dark urine.
After a run in-period of 2 weeks from screening, the subjects were randomly assigned to two groups. Group A received 20 mg of atorvastatin every alternate day while group B received the same dose of atorvastatin every day. Fasting plasma lipid profile was measured on the 6th and 12th weeks after which both the groups underwent a 4-week wash-out period. Starting from the 16th week, group A was crossed over to everyday 20 mg atorvastatin therapy whereas group B to the same treatment every alternate day. The fasting plasma lipid profile was estimated again on 16th, 22th, and 28th weeks. Serum ALT and AST were measured for both groups on 6th, 12th, 16th, 22nd, and 28th weeks. Additionally serum AST, ALT and CPK were measured if any subjects complained of myalgia any time during the course of the study. All subjects were provided with a printed checklist and a pill-box to help assessment of compliance.
This study was approved and monitored by the Ethics Committee of R. G. Kar Medical College, Kolkata.
Due to crossover design, the statistical comparison was based on within-patient differences of the study parameters between daily and alternate-day dosing periods. No significant period effect or carry-over effect was observed after the washout and the results were pooled across the periods for classical analysis of the AB/BA crossover study.[19,20] Data between the two groups were compared using the unpaired t-test. All data were presented as mean ± SEM.
The GraphPad Prism Version 5 statistical software was used in data analysis and and a P value of <0.05 was considered significant.
Results
Two patients were lost to follow-up and were considered dropouts. A total of 20 patients in group A and 18 patients in group B completed the study.
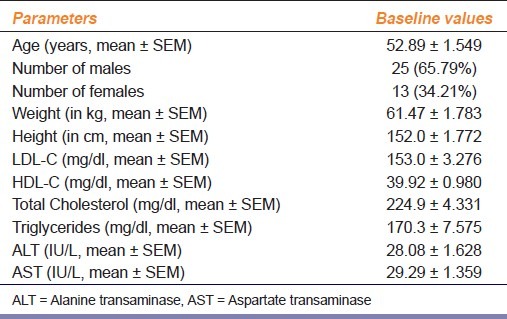
The baseline characteristics of the study population like age, sex, mean weight, mean height, plasma lipid profile, and serum ALT and AST values are represented in Table 1.
Table 1.
Baseline characteristics of the patients
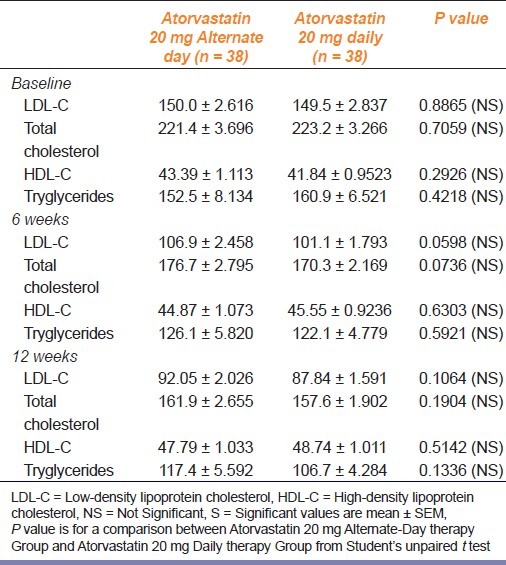
As seen in Table 2, both the regimens significantly reduced total cholesterol, LDL-C, and triglycerides after 6 weeks and 12 weeks of treatment compared to baseline. Though daily therapy with 20 mg atorvastatin caused slightly more reduction of total cholesterol, LDL-C and triglyceride, the results were statistically not significant. Likewise, there was a comparable increase in HDL-C levels in both regimens at 6 and 12 weeks from baseline.
Table 2.
Changes in LDL-C, total cholesterol, HDL –C and triglyceride in the study groups
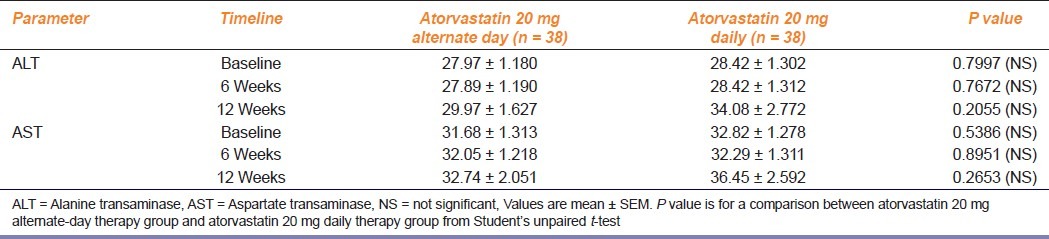
As observed from Table 3, serum ALT and AST levels were comparable in both the groups at baseline, 6, and 12 weeks, with no significant elevation (>3 times the baseline value) of the mean values of either enzymes at any point of study. However one patient on alternate-day therapy, when crossed over to daily atorvastatin therapy, complained of myalgia at second week which was associated with slight elevation of CPK above the reference value without any change in serum ALT or AST levels. The subject was subsequently shifted to alternate-day therapy and on repeat laboratory testing, the liver transaminase and CPK levels were again well within normal limits.
Table 3.
Changes in serum Alanine transaminase and Aspartate transaminase levels in the study groups
In our study, per 20 mg tablet of atorvastatin had a cost of [ ] 4.95. Therefore the cost of alternate-day therapy with the same for 12 weeks was [
] 4.95. Therefore the cost of alternate-day therapy with the same for 12 weeks was [ ] 207.9, accounting for a mean 38.37% reduction of LDL-C. The cost of treatment with daily atorvastatin for 12 weeks was [
] 207.9, accounting for a mean 38.37% reduction of LDL-C. The cost of treatment with daily atorvastatin for 12 weeks was [ ] 415.8, accounting for a mean 43.5% reduction of LDL-C. Projecting the above data to yearly expenses, the cost of treatment with every other day atorvastatin is [
] 415.8, accounting for a mean 43.5% reduction of LDL-C. Projecting the above data to yearly expenses, the cost of treatment with every other day atorvastatin is [ ] 23.48/percent reduction of LDL-C/year compared to [
] 23.48/percent reduction of LDL-C/year compared to [ ] 41.49/percent reduction of LDL-C/year with the daily therapy. This amounts to a savings of [
] 41.49/percent reduction of LDL-C/year with the daily therapy. This amounts to a savings of [ ] 18.00/percent reduction of LDL-C/year with alternate-day atorvastatin therapy over the daily treatment regimen.
] 18.00/percent reduction of LDL-C/year with alternate-day atorvastatin therapy over the daily treatment regimen.
Discussion
The results of the current study indicate that treatment with alternate-day dose of atorvastatin is comparably effective when compared to currently practiced daily atorvastatin therapy.
As evident from periodic liver enzyme estimations, we can say that alternate-day statin therapy is at least as safe as daily treatment regime. While there was one instance of myalgia, on daily atorvastatin treatment, alternate-day dosage of the same was well tolerated.
While our primary objective in this study was to compare the efficacy and safety of the two regimens, given a similar efficacy profile, prescription of the alternate-day atorvastatin doses in practice can greatly reduce the cost of therapy, bringing in some economic respite to already burdened consumers of such treatment.
Our study was not of requisite duration to evaluate the long-term benefits of alternate day statin therapy on cardiovascular profile as is applicable to the daily treatment schedule with atorvastatin from large RCTs.
All the participants of this study were selected from Medicine OPD and were treated on an outpatient basis; it is difficult to comment on whether the results of this study can be reasonably extrapolated to those requiring indoor treatment for more severe disease.
In conclusion, we can say that alternate-day treatment with atorvastatin has similar efficacy and safety compared to daily treatment with the same dose of drug; and thus is more cost effective taking into consideration the reduction in expenditure that is offered with scomparable treatment outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mukherjee AK. Prediction of coronary heart disease using risk factor categories. J Indian Med Assoc. 1995;93:312–5. [Google Scholar]
- 2.Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, O’Donnell CJ, D’Agostino RB, Massaro J, Silbershatz H, Wilson PW. Applicability of Cholesterol-lowering primary prevention trials to a General population: The Framingham Heart Study. Arch Intern Med. 2001;161:949–54. doi: 10.1001/archinte.161.7.949. [DOI] [PubMed] [Google Scholar]
- 4.Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–70. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: A double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33:226–9. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 6.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 7.Bersot PT. Drug Therapy for Hypercholesterolemia and Dyslipidemia. In: Brunton LL, editor. Goodman and Gilman's Manual of Pharmacology and Therapeutics. 12th ed. New York: McGraw-Hill; 2010. p. 893. [Google Scholar]
- 8.Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42:1141–60. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 9.WHO collaborating centre for Drug Statistic and Methodology [database on the Internet]. ATC classification index with DDDs 2011. [Cited 2011 Sep 8]. Available from: http://www.whocc.no/atc_ddd_index/?code = C10AA05. Last updated 2010-12-21 .
- 10.Roy V, Rewari S. Ambiguous drug pricing: A physician's dilemma. Indian J Pharmacol. 1998;30:404–7. [Google Scholar]
- 11.Singal GL, Nanda A, Kotwani A. A comparative evaluation of price and quality of some branded versus branded-generic medicines of the same manufacturer in India. Indian J Pharmacol. 2011;43:131–6. doi: 10.4103/0253-7613.77344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao AC, Keshava R, Patil P, Patil S, Nair R, Mammen K, et al., editors. Current Index of Medical Specialities update-4. Bengaluru: CMP Medica; 2011. Cardiovascular and hematopoietic system; pp. 108–9. [Google Scholar]
- 13.Park K. Epidemiology of chronic non-communicable diseases and conditions. In: Park K, editor. Park's Textbook of Preventive and Social Medicine. 20th ed. Jabalpur: Bhanot; 2009. p. 316. [Google Scholar]
- 14.Juszczyk MA, Seip RL, Thompson PD. Decreasing LDL cholesterol and medication cost with every-other-day statin therapy. Prev Cardiol. 2005;8:197–9. doi: 10.1111/j.0197-3118.2005.04404.x. [DOI] [PubMed] [Google Scholar]
- 15.Ebrahimi R, Jafari M, Ahmadi-Kashani M, Balian H, Bashir M. Efficacy of Alternate-Day Dosing Versus Daily Dosing of Atorvastatin. J Cardiovasc Pharmacol Ther. 2003;8:123–6. doi: 10.1177/107424840300800205. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-García JC, Pérez-Silvestre J, Martínez-Mir I, Herrera-Ballester A. Alternate-day dosing of atorvastatin: Effects in treating type 2 diabetic patients with dyslipidaemia. Acta Diabetol. 2006;43:75–8. doi: 10.1007/s00592-006-0216-4. [DOI] [PubMed] [Google Scholar]
- 17.Mangin EF, Robles GI, Jones WN, Ford MA, Thomson RS. Comparing hyperlipidemia control with daily versus twice-weekly simvastatin. Ann Pharmacother. 2004;38:1789–93. doi: 10.1345/aph.1E134. [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 19.Hills M, Armitage P. The two-period crossover clinical trial. Br J Clin Pharmacol. 1979;8:7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BW. The crossover experiment for clinical trials. Biometrics. 1980;36:69–79. [PubMed] [Google Scholar]


