Abstract
Some factors have been identified as contributing to medical errors, such as labels, appearance and location of ampoules. We present a case of accidental injection of tranexamic acid instead of Bupivacaine during spinal anaesthesia. One minute after the injection of 3 mL of the solution, the patient developed myoclonus of her lower extremities. Accidental intrathecal injection of the wrong drug was suspected and a used ampoule of tranexamic acid was discovered in the trash can. The ampoules of Bupivacaine (5 mg/mL, trade name “Sensovac Heavy”) and tranexamic acid (500 mg/mL, Trade name “Nexamin”) were similar in appearance. Her myoclonus was successfully treated with phenytoin, sodium valproate, thiopental sodium infusion, midazolam infusion and supportive care of haemodynamic and respiratory systems. The surgery was temporarily deferred. The patient's condition progressively improved to full recovery.
Keywords: Myoclonic jerks, spinal anaesthesia, tranexamic acid
INTRODUCTION
It is estimated that medication errors occur in 1.5 million people in the USA annually. In other industrialized countries, it has been reported that adverse events from drugs are a leading cause of injury and death within their health care systems.[1] In this case report, we report an accidental injection of tranexamic acid instead of hyperbaric Bupivacaine for spinal anaesthesia due to confusion between two different ampoules.
CASE REPORT
A 37-year-old female weighing 60 kg, American Society of Anaesthesiologists I physical status, was scheduled for cystolithotripsy. There was no past history of convulsions or anaesthesia exposure. Her vital parameters and investigations were within normal limits. Spinal anaesthesia was performed with the patient in the right lateral position at the L 4-5 interspace using a 23 gauge Quincke's spinal needle. Injection Bupivacaine 0.5% (15 mg) 3 mL was injected intrathecally. Immediately after drug administration, when she was placed in the supine position, she complained of severe burning pain in both lower limbs, back and gluteal region, and was irritable. She developed myoclonic movements in the lower extremities. Intravenous sedation with midazolam (2 mg) and fentanyl (100 mcg) was administered without desired effect. Inj. propofol 80 mg was given with temporary effect followed by sodium thiopentone 350 mg. The patient developed apnoea and the trachea was intubated with a 7-mm cuffed portex endotracheal tube easily. Her pulse rate was 98/min and blood pressure (BP) gradually increased to 170/98 mmHg from 110/70 mmHg. With the persistence of myoclonic jerks, IV diazepam 10 mg and Inj. atracurium 25 mg was given followed by Inj. midazolam infusion at the rate of 1.5 mg/h.
Accidental intrathecal injection of the wrong drug was suspected and a used ampoule of tranexamic acid was found in the trash can. After consultation with the neurologist, IV phenytoin 700 mg, dexamethasone 8 mg, sodium valproate 500 mg and mannitol 20% 100 mL were administered. To reduce the concentration of the drug in the cerebrospinal fluid (CSF), CSF lavage with 20 mL of normal saline was performed (30 min after intrathecal injection). Myoclonic jerks appeared in the upper limbs and face and, therefore, Inj. Atracurium infusion was started at a rate of 30 mg/h. The patient developed tachycardia (pulse rate 140–160/min) with ventricular premature beats (8–10/min) and hypertension (BP between 160/100 mmHg and 210/120 mmHg). Inj. Lignocaine 2% 3 mL was given and nitroglycerine infusion was started for the control of BP. A nasogastric tube, Foley's catheter, central venous line and arterial line were secured. Arterial blood gas (ABG) analysis revealed metabolic acidosis with pH 7.291, PaO2 300.3, PaCO2 34.7, HCO3 16.4 and BE –9.3 with SpO2 99.9%. Inj. Sodium bicarbonate 7.5% 50 mL was administered. The operation was postponed.
After stabilization of vital parameters, she was shifted to the Intensive Care Unit (ICU) about 2 h after the intrathecal injection. She was ventilated with synchronized intermittent mandatory ventilation-pressure control mode with an inspiratory pressure of 20 cmH2O, respiratory rate of 14/min and positive end-expiratory pressure of 5 cmH2O. Half an hour after shifting to the ICU, tonic clonic convulsions appeared again, midazolam infusion was increased to 3 mg/h, atracurium infusion was increased to 40 mg/h and thiopental infusion at the rate of 3–5 mg/kg/h was started. Nitroglycerine infusion was titrated to keep the BP less than 140/90 mmHg. ABG was performed at 6-hourly intervals and a total 150 mL of sodium bicarbonate at a rate of 10 mL/h was required to keep a base excess of less than –10. Inj. sodium valproate 100 mg 12-hourly, Inj. dexamethasone 8 mg every 8 h and Inj. Mannitol 20% 100 mL 8-hourly were continued and Inj. phenytoin was replaced with Inj. levetiracetam 1 gm.bd as per the advice of the neurophysician.
On the 2nd day, myoclonic jerks had stopped and therefore thiopental, atracurium and midazolam infusions were stopped sequentially. Inj. levetiracetam, valproate, dexamethasone and mannitol were continued and gradually tapered. Nitroglycerine infusion was withdrawn as her haemodynamic parameters had stabilized. ABG showed pH 7.428, PaO2 183.7, PaCO2 24.4, HCO3 15.8, BE –6.9, Na 139 and K 2.9. Sodium bicarbonate infusion was stopped and potassium chloride (KCL) infusion was started (15% 10 mL KCL in 500 mL of dextrose–saline at a rate of 100 mL/h) till the potassium came to a normal level. Hepatic, renal and haematological investigations were done for assessing organ function, which were within normal limits.
Over the next 2 days, the patient's level of consciousness gradually improved, she moved all four limbs and started responding to verbal commands. Anticonvulsants were stopped, dexamethasone was continued in a tapering dose, ventilatory support was withdrawn and she was extubated on the 5th day. She communicated normally, vitals were stable and neurological examination was normal. A computed tomography scan showed mild cerebral oedema. On the 7th day, the patient was discharged from the hospital without any neurological sequelae.
DISCUSSION
Some factors that have been identified as contributing to medication errors include labels, appearance and location of ampoules, syringes, inattention, poor communication, carelessness and fatigue on the part of the anaesthesiologist.[1] In this case, drug error-induced polymyoclonus occurred due to injection of tranexamic acid for spinal anaesthesia, as two different ampoules had a similar appearance. This illustrates the importance of double-checking to reduce such errors.
Little is known about the effect of direct intrathecal administration of tranexamic acid in humans. Wong et al.[2] reported the first case of inadvertent intrathecal injection of 75 mg tranexamic acid in an 18-year-old man scheduled for appendicectomy. He developed clonic convulsions that progressed to a generalized seizure, which treated with intravenous diazepam, and the patient recovered without any sequelae. De leede et al.[3] have reported a case of a 68-year-old man who accidentally received an intrathecal injection of 50 mg. tranexamic acid. Immediately after the injection, he developed status epilepticus. The outcome was complicated, with hypotonic paresis of all four limbs, which resolved but resulted in residual bilateral peroneal palsy. Yeh et al. reported that seizures and refractory ventricular fibrillation after accidental intrathecal administration of 500 mg tranexamic acid were associated with fatal outcome.[4] The exact mechanism by which tranexamic acid induces seizures or ventricular fibrillation is not known. There are, however, reports of neurotoxicity in experimental studies, and when applied topically to the cerebral cortex in animal studies, this drug is known to produce seizures.[5] Myoclonus could arise from cerebral cortex, brain stem, spinal cord, peripheral nerve and spinal roots. Some authors have linked it to cerebellar dysfunction. There are two types of myoclonus arising from the spinal cord; spinal segmental myoclonus and propriospinal myoclonus. In the propriospinal myoclonus, the first muscle activated is usually from the thoracic cord, with upward and downward spread resulting in generalized myoclonus. In an experimental study, the drug caused intracranial and systemic hypertension and epilepsies.[5] Very high doses of drugs would cause massive sympathetic discharge, as evidenced by initial hypertensive response and subsequent ventricular arrhythmias reported in some patients.[4]
In our case, the patient reported severe back pain and myoclonus developed immediately after the intrathecal injection of 300 mg tranexamic acid, which was a much larger dose compared with those in former case reports. Yamamura et al. reported that intracisternal injection of tranexamic acid at a dose of 5 mg/kg in cat causes seizure activities within 45–60 s.[5] In the present case, the patient received the same dose of tranexamic acid. Tranexamic acid -induced seizures result either from direct cerebral ischaemia secondary to decrease in regional or global cerebral blood flow or blockage of inhibitory cortical-amino butyric acid (GABA)-A receptors. Because GABA-A receptors govern opening of chloride channels resulting in neuronal hyperpolarisation and reduced excitability, blockage by tranexamic acid results in lowering of depolarization threshold and enhanced excitotoxicity.[6,7] Phenytoin did not terminate such severe polymyoclonus, but addition of sodium valproate was effective.[1] Refractory ventricular fibrillation by intrathecal injection of tranexamic acid that did not respond to full resuscitation is also reported.[5] Patients experiencing fatal catastrophies after incidental intrathecal injection of penicillin, gallamine and vincristine were treated with anticonvulsant and spinal fluid lavage.[7]
We speculate that treatment should include administration of anticonvulsants, intensive haemodynamic monitoring and, possibly, CSF lavage. The benefits of CSF lavage are that they remove and dilute the injected drug thus limiting the possibility of neurological damage.[8]
Finally, all these case reports were due to confusion between hyperbaric Bupivacaine and tranexamic acid. The ampoules of tranexamic acid and Bupivacaine were similar in appearance [Figure 1], but all these publications have led the manufacturer to change the configuration of ampoule of hyperbaric Bupivacaine so that such serious complications do not happen again.
Figure 1.
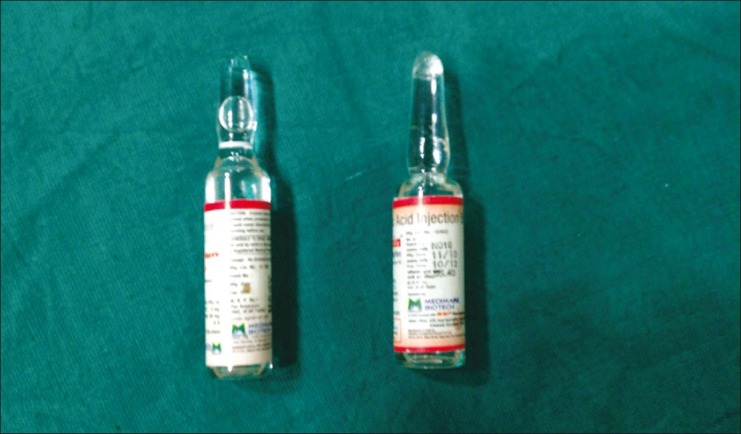
Ampoulles of Bupivacaine and tranexamic acid showing similarity in appearance
In most of the reports[1,2,4], general anaesthesia was given after spinal anaesthesia as there was no sensory and motor blockade with spinal anaesthesia. In these[1,2,4] patients, convulsions appeared after reversal of muscle relaxants. Therefore, intrathecal drug remained in the CSF for 2–3 h and treatment was started later. While in our case, treatment of convulsions and CSF lavage were performed earlier, leading to a favourable outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mohseni K, Jafari A, Nobahar MR, Arami A. Polymyoclonus seizures resulting from accidental injection of tranexamic acid in spinal anesthesia. Anaesth Anal. 2009;108:1984–6. doi: 10.1213/ane.0b013e3181a04d69. [DOI] [PubMed] [Google Scholar]
- 2.Wong J, Yang S, Tsai M. Accidental injection of tranexamic acid during spinal anaesthesia (in Chinese) Ma Zui Xue Za Zhi. 1988;26:249–52. [PubMed] [Google Scholar]
- 3.De Leede-van der, Maarl MG, Hilkens P, Bosch F. The epileptogenic effect of tranexamic acid. J Neurol. 1999;246:843. doi: 10.1007/s004150050466. [DOI] [PubMed] [Google Scholar]
- 4.Sabzi F, Teimouri H, Zokai A. Myoclonus, seizure and ventricular fibrillation after intrathecal injection of tranexamic acid. J Tehran Univ Heart Center. 2009;4:253–5. [Google Scholar]
- 5.Yeh HM, Lau HP, Lin PL, Sun WZ, Mok MS. Convulsions and refractory ventricular fibrillation after intrathecal injection of a massive dose of tranexamic acid. Anesthesiology. 2003;98:270–2. doi: 10.1097/00000542-200301000-00042. [DOI] [PubMed] [Google Scholar]
- 6.Kaabachi O, Eddhif M, Rais K, Zaabar MA. Inadvertent intrathecal injection of tranexamic acid. Saudi J Anaesth. 2011;5:90–2. doi: 10.4103/1658-354X.76504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcha PS, Mohan CV, Sharma RM. Death after an inadvertent intrathecal injection of tranexamic acid. Anesth Analg. 2007;104:241–2. doi: 10.1213/01.ane.0000250436.17786.72. [DOI] [PubMed] [Google Scholar]
- 8.Tsui BC, Malherbe S, Koller J, Aronyk K. Reversal of an unintentional spinal anesthetic by cerebrospinal lavage. Anesth Analg. 2004;98:434–6. doi: 10.1213/01.ANE.0000095152.81728.DC. [DOI] [PubMed] [Google Scholar]


