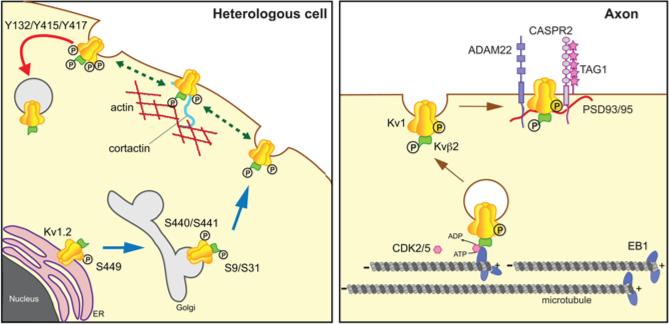
Fig. 3.
Modulation of Kv1 channels. Left panel. Studies in heterologous cells have revealed diverse roles for phosphorylation of both Kv1.2 and Kvβ2, including ER export, forward trafficking to the plasma membrane, association with the actin-based cortical cytoskeleton, and endocytosis. S9 and S31 refer to phosphorylation sites on Kvβ2, and Y132, Y415, Y417, S440/S441, and S449 on Kv1.2. Right panel. Altering phosphorylation of Kvβ2 leads to changes in binding to EB1 and dissocation of Kv1 channel complexes from MTs, allowing for local insertion into the plasma membrane and direct or indirect association with interacting proteins ADAM22, CASPR2, PSD93/95, and TAG1.