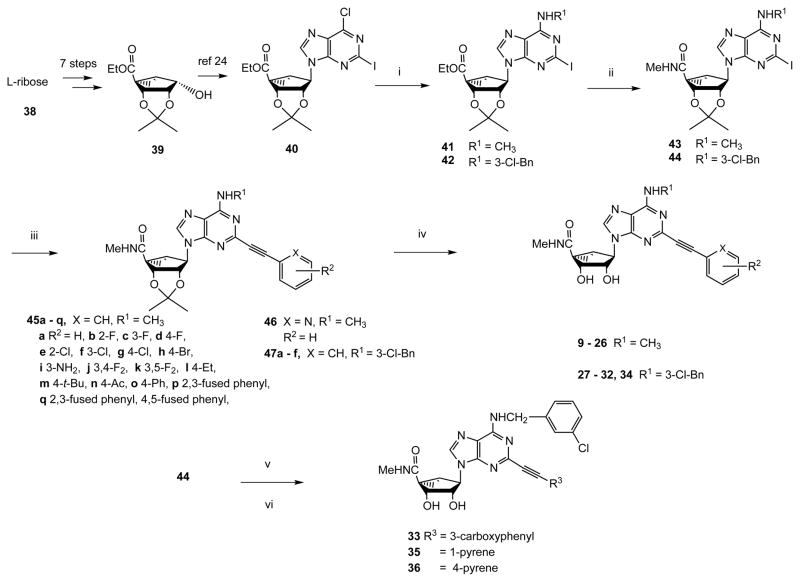
Scheme 1.
Synthesis of (N)-methanocarba nucleoside analogues. R1 = Me or 3-Cl-benzyl. Reagents: (i) MeNH2 or 3-Cl-benzylamine, Et3N, MeOH, rt; (ii) 40% MeNH2, MeOH, rt; (iii) HC≡CAr, Pd(PPh3)2Cl2, CuI, Et3N, DMF, rt; (iv) 10% TFA, MeOH, 70°C; (v) 1-ethynylpyrene or 4-ethynylpyrene 51, Pd(PPh3)2Cl2, CuI, Et3N, DMF, rt; (vi) HCl, 1 N, dioxane, 60°C.