Fig 7.
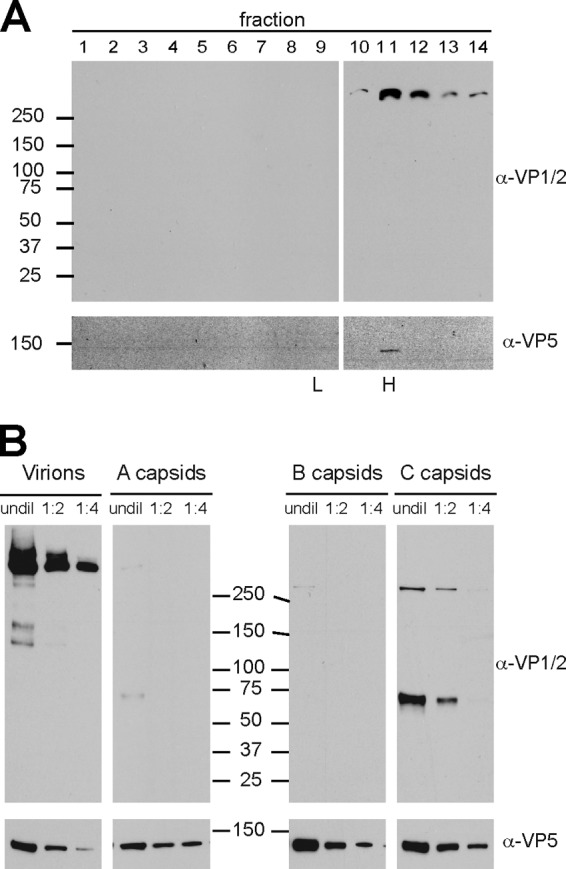
Relative incorporation levels of VP1/2 isoforms onto nuclear capsids and extracellular viral particles. (A) Extracellular viral particles from PK15 cells infected with wild-type PRV were separated through a 12 to 32% dextran gradient. Fractions were collected from the top, TCA precipitated, and blotted with antibodies directed against VP1/2 and the VP5 major capsid protein. Molecular size markers (in kDa) are indicated at left; peak fractions containing heavy (H) and light (L) viral particles observed as light-scattering bands in the gradient are indicated at bottom. (B) Heavy particles (virions) from fraction 11 of panel A were serially diluted 2-fold alongside preparations of intranuclear A, B, and C capsids from PK15 cells infected with wild-type PRV. The A, B, and C capsid light-scattering bands were harvested as three discrete fractions from a 20 to 50% sucrose gradient. The membranes were probed with antibodies directed against VP1/2 and the VP5 capsid protein, the latter serving as the loading control. Molecular size markers (in kDa) are shown down the middle to indicate the relative migration of the samples across two gels. undil, undiluted.
