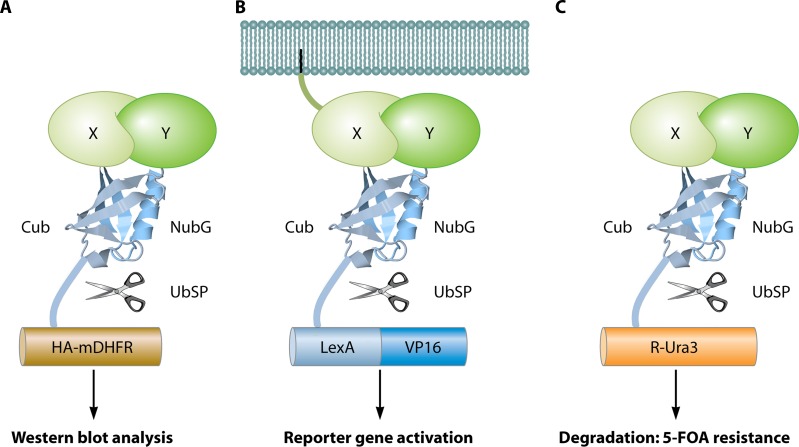
Fig 11.
The split-ubiquitin system. (A) mDHFR-HA readout (312). Bait protein X and prey protein Y are fused to the C-terminal (Cub) and mutated (I13G) N-terminal (NubG) domains, respectively, of ubiquitin. In addition, a chimera of mDHFR and the HA epitope completes the bait construct. Upon interaction of X with Y, a fully reconstituted ubiquitin is recognized by ubiquitin-specific proteases (UbSP) that cleave HA-mDHFR, resulting in a shift on a Western blot using anti-HA antibodies. (B) LexA-VP16 readout (611). Interaction between a membrane-bound bait X and prey Y leads to cleavage of the artificial transcription factor LexA-VP16. Released LexA-VP16 localizes to the nucleus to activate reporter genes. (C) R-Ura readout (372). Interaction between bait X and prey Y induces the release of Ura3 (with an N-terminal arginine) by proteases. Exposed arginine makes Ura3 highly unstable, resulting in its degradation. Due to the strongly reduced concentration of Ura3, the cells become viable on medium with 5-FOA, a prototoxic substrate of Ura3. The image of the ubiquitin protein is based on the PDB structure under accession number 1UBQ (671).