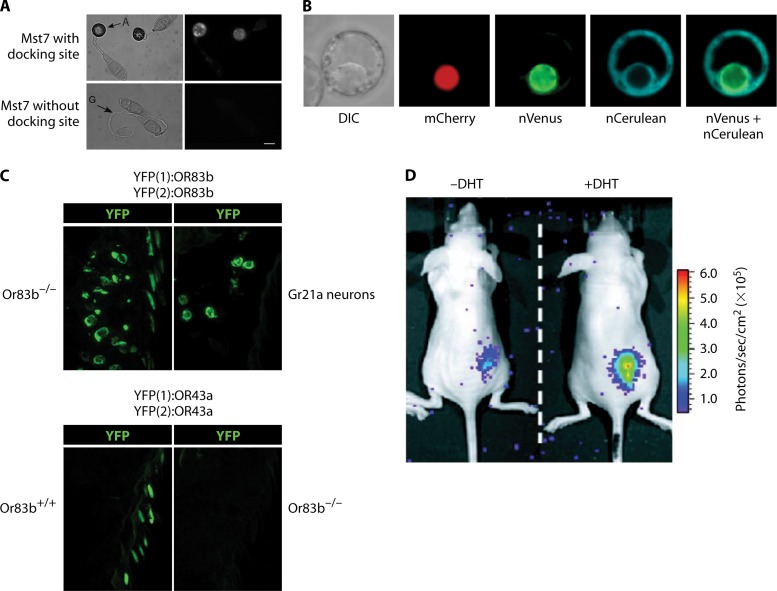
Fig 13.
Visualization of PPIs by PCAs. (A) Split-YFP assay in the plant pathogen Magnaporthe grisea (732). Pmk1 and Mst7 kinases, which are components of the MAP kinase pathway essential to appressorium formation and plant infection, were fused to the C-terminal and N-terminal parts of YFP, respectively. Interaction between Pmk1 and Mst7 was observed in vivo in appressorium formations only when the putative docking site of Mst7 was intact. A, appressorium; G, germ tube. (Adapted from reference 732 with permission.) (B) Multicolor split-FP assay in tobacco culture cells. Protoplasts were transfected by direct DNA uptake and visualized using laser scanning confocal microscopy. Simultaneous interactions between Agrobacterium tumefaciens VirE2 (VirE2-cCFP) and the Arabidopsis nuclear transport adapter protein importin α-1 (Impa-1-nCerulean) and between VirE2 and importin α-4 (Impa-4-nVenus) were observed in the cytoplasm (cerulean) and nucleus (Venus), respectively. Nuclear localization was confirmed by colocalization of the nuclear marker mCherry-VirD2NLS. Labels below each image indicate the filter set/channel imaged. DIC, differential interference contrast image. (Adapted from reference 375 with permission.) (C) Visualization of odor-evoked calcium release upon formation of functional heteromeric complexes of odorant receptors (ORs) in Drosophila, using a split-YFP assay. (Top) Complementary N-terminal and C-terminal fragments of YFP [YFP(1) and YFP(2)] were fused to the odorant receptor OR83b. (Left) Dimerization of OR83b in neurons lacking native OR83b, visualized by the split-YFP method. (Right) Dimerization of OR83b is still visible in Gr21a neurons, which do not express any native ORs, suggesting a direct PPI. (Bottom) Complementary N-terminal and C-terminal fragments of YFP [YFP(1) and YFP(2)] were fused to the odorant receptor OR43a. YFP complementation is visible in neurons with OR83b but not in neurons lacking OR83b. This implicates that OR43a dimerization depends on the presence of OR83b and may not be a direct PPI. (Adapted from reference 38 with permission.) (D) In vivo imaging of split Renilla luciferase (RLuc) complementation in living mice (344). The strategy to monitor translocation of a particular protein into the nucleus is based on reconstitution of split RLuc by the intein Dna-E (also see Fig. 12H). RLuc-N (N-terminal part) was fused to DnaE-N and a nuclear localization signal. This chimera localizes mainly to the nucleus. RLuc-C (C-terminal part) was fused to DnaE-C and a protein of interest, the nuclear androgen receptor (AR), which localizes to the cytosol. Translocation of AR into the nucleus was visualized upon addition of 5a-dihydrotestosterone (DHT), which binds AR, in COS-7 cells implanted on the backs of mice. DHT-induced translocation of AR results in reconstitution of the DnaE intein and its splicing-reassembly property. Consequently, the spliced and reconstituted RLuc recovers its bioluminescence activity, which is imaged by using a cooled CCD camera and measured as photons per second per cm2. The differential translocation of AR in the presence (+) or absence (−) of DHT could hence be evaluated quantitatively. (Adapted from reference 344 [copyright 2004, National Academy of Sciences].)