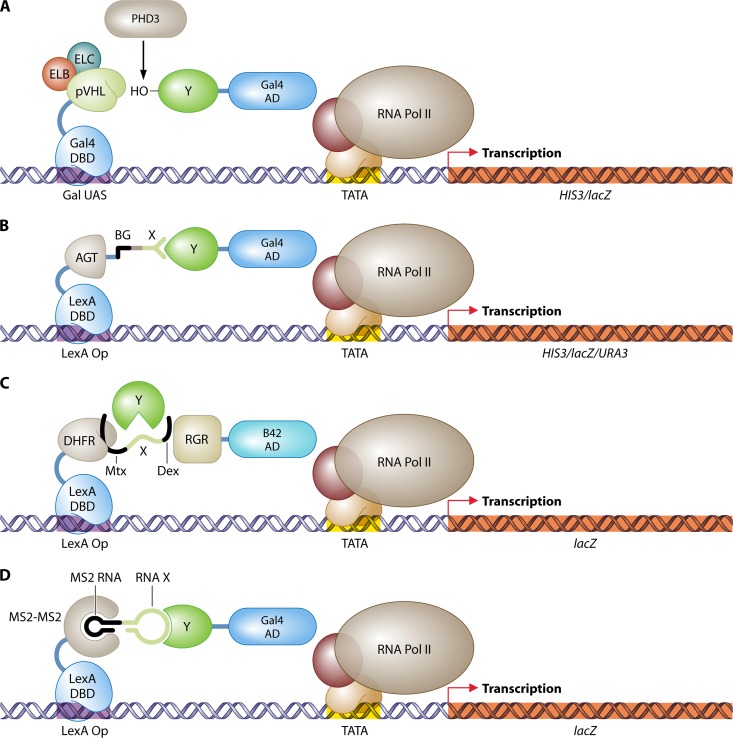
Fig 7.
Three-hybrid systems. (A) Identification of target proteins of the E3 ubiquitin ligase complex CBCVHL (41). The pVHL subunit is fused to the LexA DBD, and coexpression of elongins B and C (ELB and ELC) stabilizes the native conformation of pVHL. A human cDNA library in fusion with the Gal4 AD is screened for interactions with pVHL. The human prolyl hydroxylase PHD3 delivers the hydroxyl group to interacting prey proteins, which is essential for recognition by pVHL. (B) Protein–small-molecule interactions (94). O6-Alkylguanine-DNA alkyltransferase (AGT) is fused to the Gal4 DBD and can bind covalently with the small molecule O6-benzylguanine (BG) in vivo. A library of BG small-molecule heterodimers, produced in vitro, can be screened for interactions with a prey protein Y attached to the Gal4 AD. (C) Detection of enzymatic substrate recognition (21). A chimeric protein of LexA and DHFR binds the promoter region of the reporter gene lacZ and interacts with a tripartite small molecule through association of DHFR with Mtx. This small-molecule trimer further consists of a bait linker X and dexamethasone (Dex). Dexamethasone interacts with a fusion of the rat glucocorticoid receptor (RGR) and the B42 AD. The whole complex stimulates expression of lacZ. An enzyme Y that targets and cleaves linker X can be identified by disruption of the transcription activating complex and the loss of lacZ expression. (D) RNA-protein interactions (265). The LexA DNA-binding domain is fused to a head-to-tail dimer of the RNA-binding protein MS2. This hook protein associates with an RNA dimer of bacteriophage MS2 RNA and a bait RNA stretch (X). Interaction of this RNA X with a protein Y fused to the Gal4 AD is detected by stimulation of reporter gene expression. DBD, DNA-binding domain; AD, activation domain; UAS, upstream activating sequence; Op, operator; Pol, polymerase.