Fig 1.
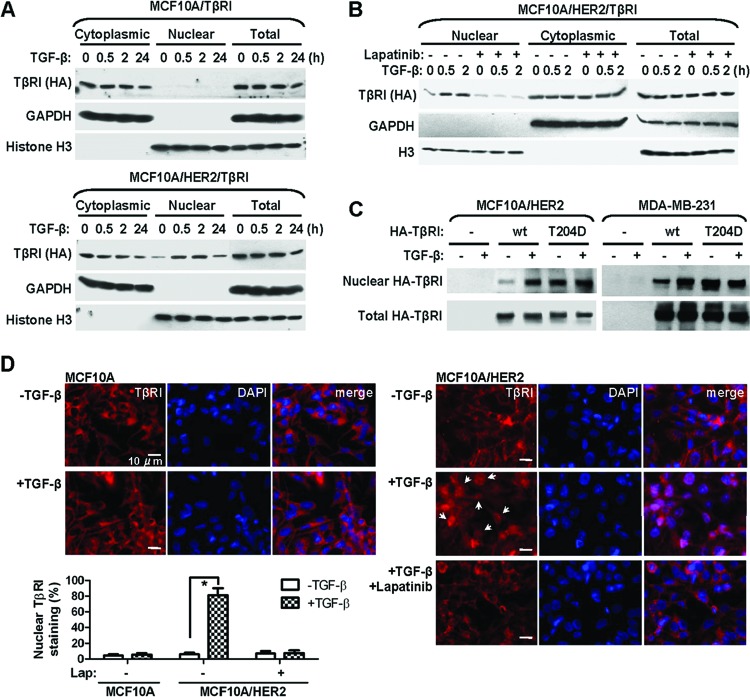
Nuclear transport of TβRI is ligand inducible and requires a transformed context. (A) Cytoplasmic and nuclear fractions were prepared from MCF10A/TβRI and MCF10A/HER2/TβRI cells that were treated with TGF-β (2 ng/ml) for the indicated times. Western blotting was carried out using the indicated antibodies. Purity of different cellular fractions was confirmed by Western blotting of GAPDH and histone H3, which are exclusively located in the cytoplasm and nucleus, respectively. (B) MCF10A/HER2/TβRI cells were treated with lapatinib (1 μM) for 6 h before TGF-β treatment. Cell fractionation and Western blotting were performed as indicated. (C) Cells were transfected with vector, wild-type (wt) TβRI construct, or a constitutively active TβRI construct (T204D) as indicated. At 24 h after transfection, cells were treated with TGF-β for 0.5 h or left untreated, and they were then subjected to fractionation and Western blotting. (D) IFA images of MCF10A and MCF10A/HER2 cells treated with TGF-β for 0.5 h or left untreated, in the presence or absence of a prior 6-h lapatinib treatment. Cells were stained with TβRI antibody for detection of endogenous TβRI (red) and 4′,6-diamidino-2-phenylindole (DAPI) for nuclei (blue). The bar equals 10 μm. Nuclear TβRI is indicated by arrows. The graph at the bottom shows percentages of cells with nuclear staining of TβRI. Two hundred cells under each treatment were counted, and the average of three independent experiments is shown. *, P < 0.001.