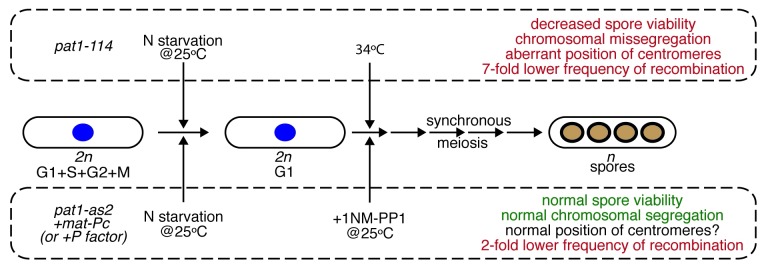
Meiosis represents a fundamental process involved in the formation of haploid gametes from diploid cells during sexual reproduction of eukaryotic organisms. It was discovered in the second half of 19th century and, after pioneering studies of Thomas H. Morgan in the 1910s, has become a hot topic of countless genetic studies. In spite of more than a century’s worth of research on meiosis, many of its crucial features remain unknown. For example, one of the highlights of first meiotic division (meiosis I) is resistance of centromeric cohesion to cleavage by separase. Although it is clear that this is an essential prerequisite for successful segregation of homologous chromosomes, its molecular nature is not fully understood.1 A model organism particularly suitable for investigating meiosis-related molecular mechanisms is fission yeast Schizosaccharomyces pombe. Vegetative diploid cells of this yeast readily undergo meiotic program when starved for nitrogen and (unlike budding yeasts) produce ordered tetrads that allow direct determination of segregation during the first and second meiotic divisions. However, as investigations of events accompanying meiosis require highly synchronous cell populations, naturally sporulating cells exhibiting a low level of synchrony are not suitable for systematic analyses. For more than two decades, these studies relied on the use of thermosensitive pat1–114 allele. The wild-type pat1+ gene encodes protein kinase Pat1/Ran1, which negatively controls the initiation of meiosis.2-4 Upon induction at non-permissive temperature, nitrogen-starving pat1–114/pat1–114 cells progress through the meiotic program in a highly synchronous manner. Although the meiosis in the pat1–114 mutant is similar to that of wild-type cells, non-permissive temperature itself has adverse effects. For example, the cells cultivated at elevated temperature exhibit aberrant centromere positioning, chromosome missegregation, substantial reduction of recombination and poor viability of spores (Fig. 1). Therefore, synchronizing meiotic cells at physiological temperature is highly desired. To address this problem, Cipak et al.5 and Guerra-Moreno et al.7 took advantage of chemical genetics. They used an approach based on conditional inactivation of target protein kinase by cell-permeable ATP analogs (1-NM-PP1 and 3-MB-PP1).6,8,9 Cipak et al.5 constructed the pat1-as2 (ATP analog-sensitive) allele coding for the Pat1 kinase with altered ATP-binding pocket. The mutant kinase Pat1(L95A) can be easily inactivated by addition 1-NM-PP1 to cultivation media, enabling the induction of the meiotic program at physiological temperature. As expected, the level of synchrony in the pat1-as2 population is similar to pat1–114 cells cultivated at non-permissive temperature. Importantly, the use of ATP analog-sensitive alleles eliminates some of the abnormalities observed at elevated temperature. Moreover, the authors further improved their experimental system by combining the pat1-as2 allele with activating pheromone signaling by ectopic expression of both mating-type loci. This improved fidelity of chromosome segregation, spore viability as well as recombination to levels similar to that observed in the wild-type cells (Fig. 1). Guerra-Moreno et al.7 synchronized meiosis using the Pat1(L95G) mutant that can be inactivated by 3-MB-PP1. Moreover, they found that induced cells exhibit similar meiosis-related transcription events as the pat1–114 strain (except for the transcription pattern of the stress response gene hsp9 probably due to cultivation of the pat1–114 mutant at elevated temperature).
Figure 1. Induction of synchronous meiotic cultures at physiological temperature. The standard procedure4 for obtaining synchronous meiotic cultures employs a temperature-sensitive allele (pat1–114) of the negative regulator of meiosis, protein kinase Pat1. However, due to the increased temperature the cells undergoing meiosis exhibit several abnormalities (in red). Cipak et al.5,6 prepared an ATP analog (1-NM-PP1) sensitive version of Pat1 [Pat1(L95A)] that can be inhibited at physiological temperature. When Pat1(L95A) carrying strain also contained ectopically expressed mat-Pc the authors observed a substantial improvement in most of the analyzed characteristics of meiosis (in green). In a similar study, Guerra-Moreno et al.7 synchronized meiotic cells by inactivating of Pat1(L95G) mutant by another ATP analog (3-MB-PP1).
In summary, both reports5,7 further strengthened the advantages of fission yeast as the workhorse in cell biology. The experimental tool they developed for synchronization of entire cell populations at nearly physiological conditions provides a promising venue for upcoming discoveries in the field of meiosis.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20314
References
- 1.Gregan J, et al. Cell Cycle. 2008;7:151–3. doi: 10.4161/cc.7.2.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iino Y, et al. Proc Natl Acad Sci USA. 1985;82:2447–51. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurse P. Mol Gen Genet. 1985;198:497–502. doi: 10.1007/BF00332946. [DOI] [Google Scholar]
- 4.Bähler J, et al. Curr Genet. 1991;19:445–51. doi: 10.1007/BF00312735. [DOI] [PubMed] [Google Scholar]
- 5.Cipak L, et al. Cell Cycle. 2012:1625–32. [Google Scholar]
- 6.Cipak L, et al. Cell Cycle. 2011;10:3527–32. doi: 10.4161/cc.10.20.17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra-Moreno A, et al. Cell Cycle. 2012;11:1620–4. doi: 10.4161/cc.20051. [DOI] [PubMed] [Google Scholar]
- 8.Bishop AC, et al. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 9.Gregan J, et al. Nat Protoc. 2007;2:2996–3000. doi: 10.1038/nprot.2007.447. [DOI] [PMC free article] [PubMed] [Google Scholar]