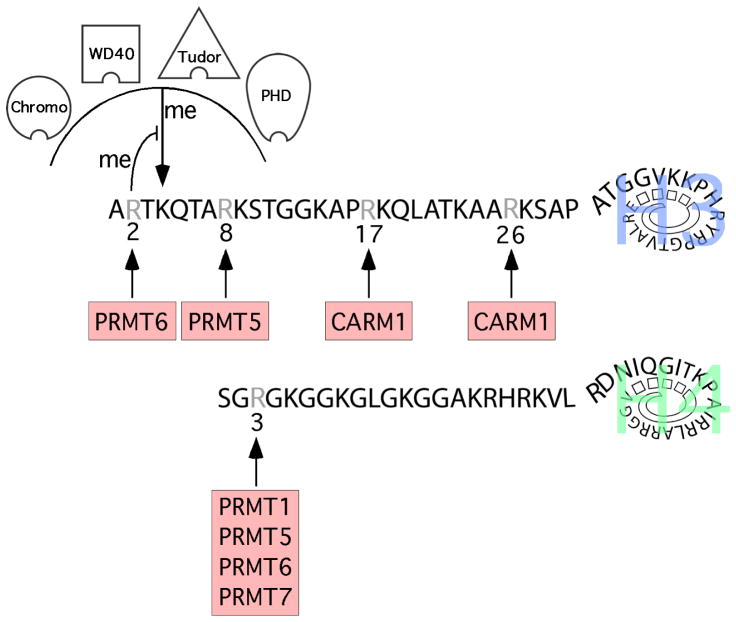
Figure 3. Arginine methylation of histone H3 and H4 N-terminal tails.
The sites of PRMT-mediated methylation on the histone tails are noted. H3R2 methylation antagonizes the docking of a number of effector proteins, including those containing chromo, PHD, Tudor, and WD40 domains. H3R2 methylation structurally impedes the binding of H3K4me3 recognition domains and also prevents H3K4 methylation by the MLL1 complex. The first 5 residues of histones H2A and H4 are identical, and H2A is also methylated at the R3 position.