Table 1.
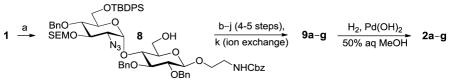
Generation of partially and fully deprotected GlcN(α1→4)Glc sulfoforms
 | ||
|---|---|---|
| sulfoforma | 9 (yield from 1, ops)b | 2 (yield from 9)c |
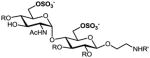 a |
52%, 6 ops (a, b, d, e, h, k) | 87% |
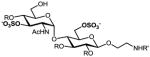 b |
39%, 6 ops (a, g, d, e, c, k) | 83% |
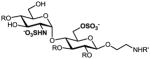 c |
36%, 7 ops (a, e, h, c, i, j, k) | 84% |
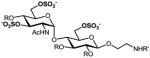 d |
48%, 6 ops (a, b, g, d, f, k) | 97% |
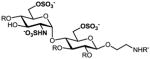 e |
42%, 6 ops (a, b, i, f, h, k) | 98% |
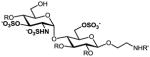 f |
40%, 6 ops (a, g, i, f, c, k) | 97% |
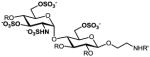 g |
54%, 6 ops (a, b, g, i, f, k) | 99% |
9a–g: R=Bn, R′=Cbz; 2a–g: R, R′=H. All products are isolated as sodium salts.
Isolated yield after HPLC purification. Each step was conducted at rt unless otherwise noted. Reagents and conditions: (a) CAN (3 equiv), 90% aq CH3CN, 0 °C, 10 h; (b) TBAF (6 equiv), THF, 6 h; (c) TBAF (8–10 equiv), 1:1 THF:DMF, 24–40 h; (d) 1:5 AcSH:Py, 48 h; (e) SO3·Me3N (20–30 equiv), DMF, 55 °C, 12 h; (f) SO3·Py (20–30 equiv), 1:5 Et3N:Py, 70 °C, microwave, 1 h; (g) MgBr2·Et2O (10 equiv.), CH3NO2 (20 equiv), Et2O, 10 h; (h) MgBr2·Et2O (10 equiv.), 1:2 CH3NO2:Et2O, 24–48 h; (i) HS(CH2)3SH (35 equiv), Et3N (35 equiv), MeOH, 24 h; (j) SO3·Py (10 equiv), 5:1 H2O:DMF, pH 9.5, 8 h; (k) Na+ ion exchange chromatography.
10% Pd(OH)2, H2 (1 atm), 1:1 MeOH:H2O, 20 h.
