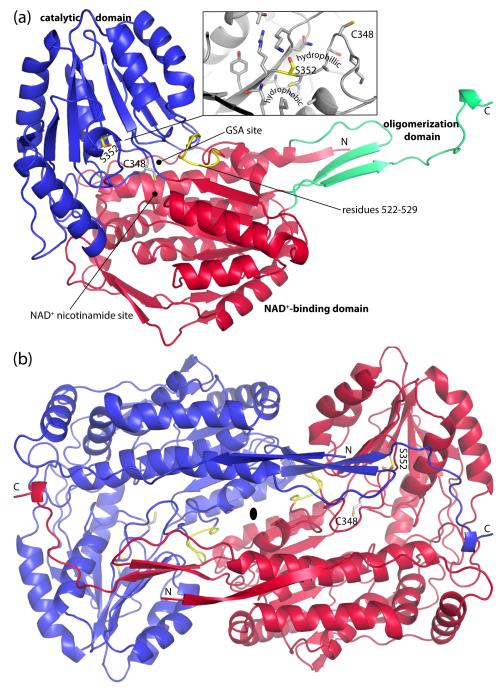
Fig. 2.
Protomer and dimer structure of HsP5CDH. (a) Ribbon drawing of the protomer. The NAD+-binding, catalytic, and oligomerization domains are colored red, blue, and green, respectively. Ser352 and residues 522-529 are colored yellow. The side chain of catalytic Cys348 is shown. Inset: close-up view of the environment around Ser352. (b) The HsP5CDH dimer. The two chains are colored red and blue. This figure and others were created with PyMOL.50