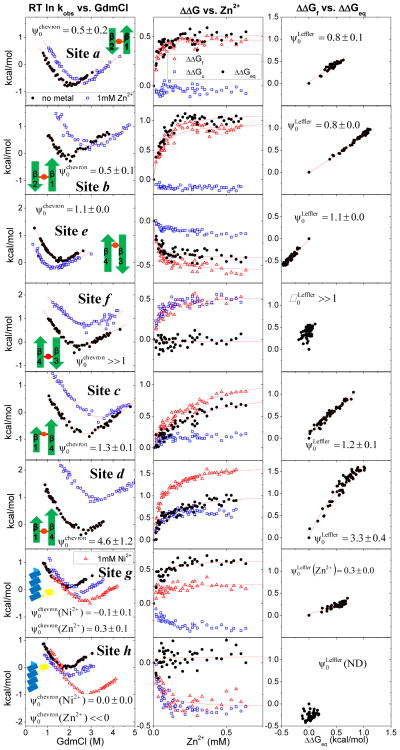
Fig. 2. Metal-dependent kinetic data. Left.
The denaturant chevrons in the absence of metal ions (black solid circle), in the presence of 1 mM Zn2+ (blue open square), and with Ni2+ (red open triangle). Center. Kinetics as a function of Zn2+ at fixed [GdmCl]. The changes in stability ΔΔGeq (black solid circle), folding activation energy ΔΔGf (red open triangle), and unfolding activation energy ΔΔGu (blue open square) plotted against Zn2+ concentration. Values are calculated by measuring the folding and unfolding rates at fixed low (~0.6 M) and high (~3.5–5 M) GdmCl concentrations, respectively (Table 2). The binding affinities are obtained from fitting to the Eq. 1. Right. Corresponding Leffler plots.