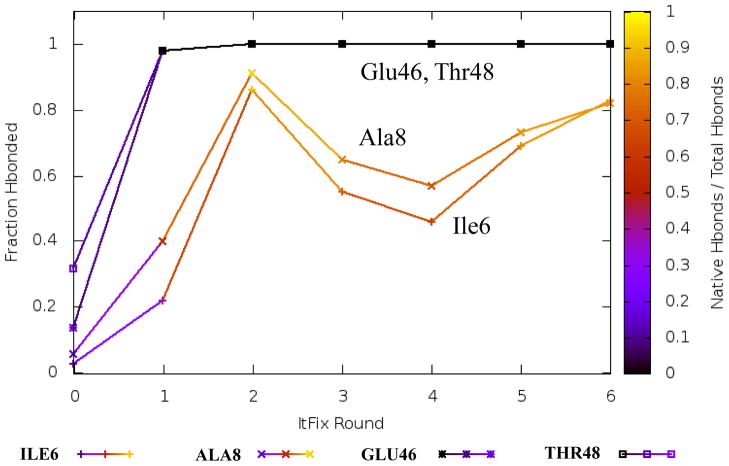
Fig. 5. Rate of β hairpin formation.
The carboxy terminal hairpin forms in fewer rounds than the amino terminal hairpin. The carboxy hairpin folds in a single round, with 98% of the structures containing hydrogen bonds between the three non-native amino acid partners. In contrast, the amino terminal hairpin requires two rounds of folding to obtain a native hairpin conformation. After the first and second rounds, 19% and 81% of the native hydrogen bonds are formed, respectively. The y-axis presents the fraction of the maximum number of hydrogen bonds for the hairpins. The color intensity reflects the native character (0=non-native, 1= all native).