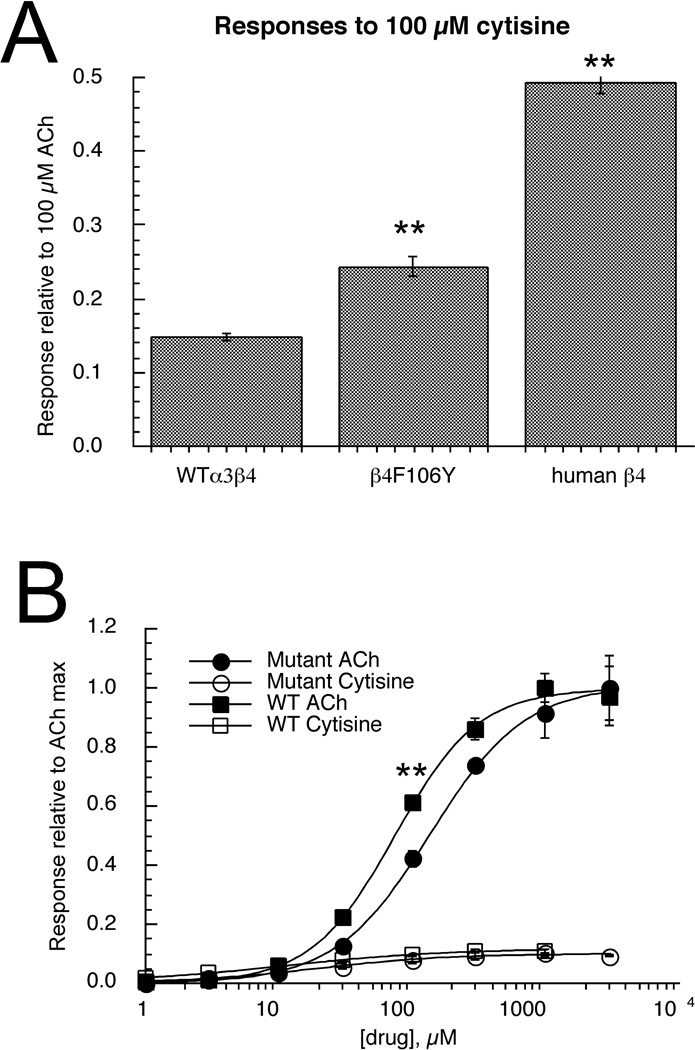
Figure 6.
The effects of the β4F106Y mutation on agonist-evoked responses of α3-containing zebrafish nAChR. A) Responses of wild-type fish α3β4 receptors to 100 µM cytisine compared to responses from oocytes expressing α3 and the β4F106Y mutant and to hybrid receptors expressing fish α3 subunits and human β4 subunits. In each case responses were measured relative to 100 µM ACh-evoked control responses. The wild-type cytisine responses were significantly less than those of either the mutant or hybrid receptors measured with this normalization procedure. B) In order to validate the normalization procedure, complete concentration-response studies were conducted for α3β4F106Y receptors for both ACh and cytisine. The data are shown compared to the wild-type data from Figures 4 (ACh) and 5B (cytisine). All of the data are normalized to the empirically determined ACh maximum responses. The main effect of the mutation appeared to be a shift in the potency of ACh, so that when expressed relative to ACh maximum, the 100 µM ACh responses of the mutants were significantly smaller (P <.001) than those of the wild-type receptors. Each bar or point is the average ± SEM of responses from at least four oocytes.