Abstract
α1-Adrenoceptors are involved in numerous physiological functions, including micturition. However, the pharmacological profile of the α1-adrenoceptor subtypes remains controversial. Here, we review the literature regarding α1-adrenoceptors in the lower urinary tract from the standpoint of α1L phenotype pharmacology. Among three α1-adrenoceptor subtypes (α1A, α1B and α1D), α1a-adrenoceptor mRNA is the most abundantly transcribed in the prostate, urethra and bladder neck of many species, including humans. In prostate homogenates or membrane preparations, α1A-adrenoceptors with high affinity for prazosin have been detected as radioligand binding sites. Functional α1-adrenoceptors in the prostate, urethra and bladder neck have low affinity for prazosin, suggesting the presence of an atypical α1-adrenoceptor phenotype (designated as α1L). The α1L-adrenoceptor occurs as a distinct binding entity from the α1A-adrenoceptor in intact segments of variety of tissues including prostate. Both the α1L- and α1A-adrenoceptors are specifically absent from Adra1A (α1a) gene-knockout mice. Transfection of α1a-adrenoceptor cDNA predominantly expresses α1A-phenotype in several cultured cell lines. However, in CHO cells, such transfection expresses α1L- and α1A-phenotypes. Under intact cell conditions, the α1L-phenotype is predominant when co-expressed with the receptor interacting protein, CRELD1α. In summary, recent pharmacological studies reveal that two distinct α1-adrenoceptor phenotypes (α1A and α1L) originate from a single Adra1A (α1a-adrenoceptor) gene, but adrenergic contractions in the lower urinary tract are predominantly mediated via the α1L-adrenoceptor. From the standpoint of phenotype pharmacology, it is likely that phenotype-based subtypes such as the α1L-adrenoceptor will become new targets for drug development and pharmacotherapy.
LINKED ARTICLE
This article is commented on by Ventura, pp. 1223–1225 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2011.01663.x
Keywords: α1L-adrenoceptor, α1A-adrenoceptor, benign prostatic hyperplasia (BPH), stress urinary incontinence (SUI), lower urinary tract, phenotype pharmacology
Introduction
The lower urinary tract is responsible for urine storage and voiding (Andersson and Wein, 2004). In mammalian species including humans, the bladder detrusor muscle contracts through a parasympathetic cholinergic mechanism during micturition (Abrams et al., 2006; Wess et al., 2007). Besides the importance of the cholinergic system as the major mechanism for bladder neck muscle tone control, the adrenergic systems play significant roles in the regulation of bladder neck tone (Fig. 1). During the storage phase, the urethra and outlet region of the bladder is contracted to maintain continence. There is good pharmacological evidence supporting the view that noradrenaline-mediated contraction of the urethral smooth muscle has an important role (Andersson and Wein, 2004).
Figure 1.
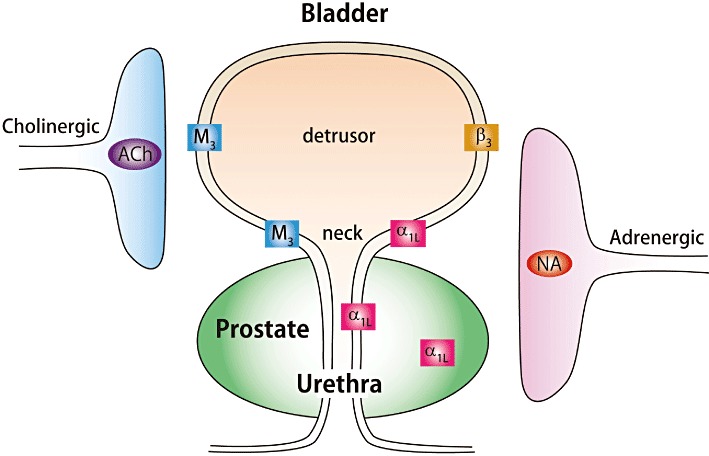
Autonomic innervation and main functional receptors involved in controlling smooth muscle tension in lower urinary tract tissues. NA, noradrenaline; α1L, α1L-adrenoceptor; β3, β3-adrenoceptor; M3, M3-muscarinic acetylcholine receptor.
Among impairments in urine storage, stress urinary incontinence (SUI) is recognized as one of the most frequently occurring conditions. Deficient urethral or bladder neck closure may result in this condition. However, drug treatments for SUI are scarce, at present (Andersson and Wein, 2004). The density of noradrenergic nerves increases markedly towards the bladder neck and urethra (Gosling et al., 1999). α1-Adrenoceptors play important roles in both urethral and bladder neck contraction, and may be related to SUI (Andersson and Wein, 2004).
During the voiding phase, prostatic smooth muscle tone contributes to outlet resistance regulation. Benign prostatic hyperplasia (BPH) is a common enlargement of the prostate gland that may lead to bladder outlet obstruction, and lower urinary tract symptoms. BPH is currently recognized as a target for pharmacotherapy utilizing α1-adrenoceptor antagonists. In BPH patients, enlargement of the prostate increases bladder outlet resistance and thereby impedes physiological voiding. α1-Adrenoceptor antagonists are believed to inhibit contraction of the prostate and urethra, and as a result, α1-adrenoceptor antagonists such as tamsulosin, alfuzosin and silodosin effectively reduce resistance to urinary flow and are now clinically used in BPH patients (Lefevre-Borg et al., 1993; Cooper et al., 1999; Ruffolo and Hieble, 1999; Takeda et al., 1999; Chapple, 2001; Andersson, 2002; Michel and Vrydag, 2006).
The functional roles of α1-adrenoceptors have been highlighted through the study of their roles in lower urinary tract physiology. These are supported by numerous studies including quantification of mRNAs, anatomical localization of mRNAs, or of receptors demonstrated by ligand-binding studies (Table 1). On the other hand, functional studies have shown discrepancies between the native α1-adrenoceptors found in the lower urinary tract (so-called α1L-adrenoceptor) and the classical α1-adrenoceptors (α1A, α1B and α1D; receptor nomenclature follows Alexander et al., 2011) (Table 2). Subsequent studies revealed the origin of these unique α1-adrenoceptors in the lower urinary tract (Ford et al., 1997; Gray et al., 2008; Muramatsu et al., 2008; Nishimune et al., 2010b). In this review, we will introduce recent progress in the study of α1-adrenoceptors in the lower urinary tract as an example of the importance of phenotype pharmacology (Kenakin, 2003; Muramatsu et al., 2005; 2008; Nelson and Challiss, 2007; Su et al., 2008).
Table 1.
α1Adrenoceptor subtypes in lower urinary tract
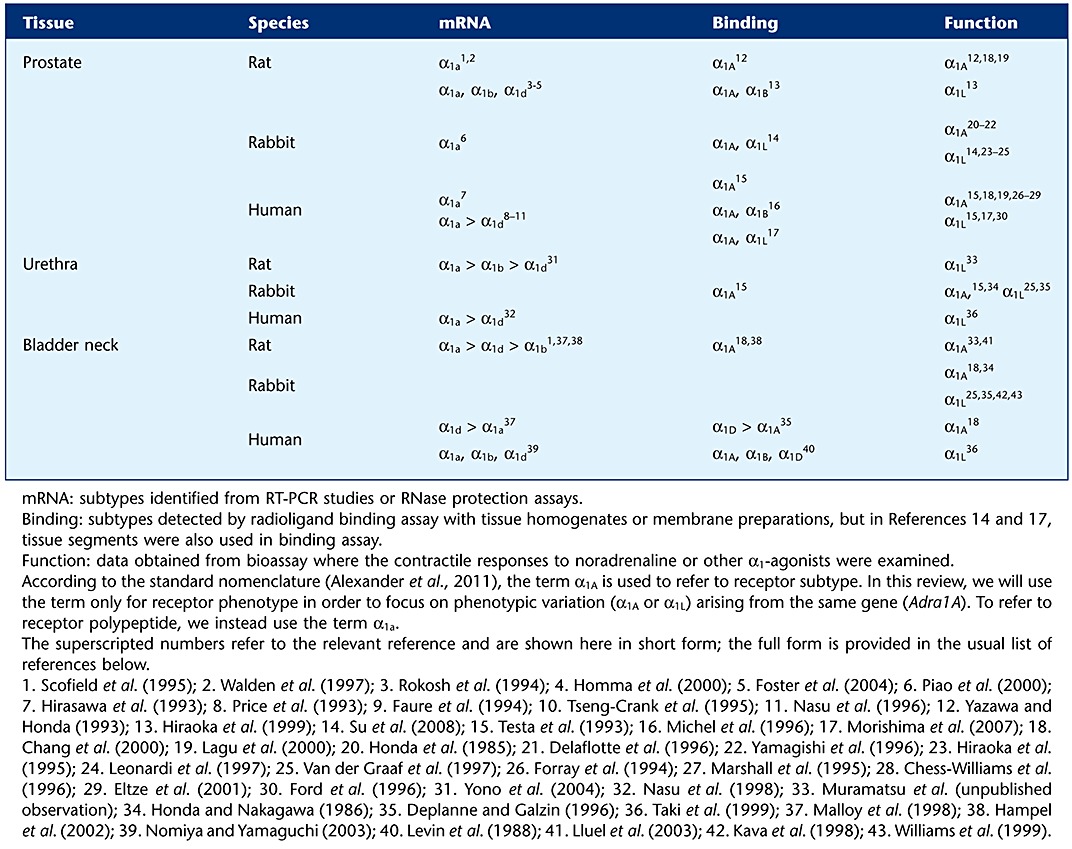 |
Table 2.
α1-Adrenoceptor subtypes and their pharmacological characterization
| Gene | Receptor polypeptide | Phenotype | Affinity (mean pKB) Prazosin | Silodosin | Tamsulosin | RS-17053 |
|---|---|---|---|---|---|---|
| ADRA1A | α1a | α1A | 9.6 | 9.8 | 10.0 | 8.7 |
| α1L | 8.0 | 9.8 | 10.0 | 6.3 | ||
| ADRA1B | α1b | α1B | 10.2 | 8.0 | 9.3 | 7.8 |
| ADRA1D | α1d | α1D | 10.0 | 8.4 | 9.9 | 7.8 |
Affinity values are from Morishima et al. (2007).
α1-Adrenoceptor mRNAs in the lower urinary tract
Three cDNAs for distinct α1-adrenoceptor subtypes (α1a, α1b and α1d) have been cloned, and their pharmacological phenotypes are consistent with pharmacological characteristics of α1-adrenoceptors found in many native tissues (Lomasney et al., 1991; Hieble et al., 1995) (Table 2). In the lower urinary tract, the mRNA and protein for these three subtypes have been demonstrated (Table 1) (Michelotti et al., 2000; Michel and Vrydag, 2006; Nishimune et al., 2010a). Among them, the α1a-adrenoceptor mRNA is the most form in the lower urinary tract of many species (Table 1). Data from human studies indicate that the relative abundance ratio of α1a- : α1b- : α1d-adrenoceptor mRNA is approximately 70:0:30 in the prostate and 90∼100:0:0∼10 in the urethra (Price et al., 1993; Faure et al., 1994; Nasu et al., 1996; 1998; Michel and Vrydag, 2006). Interestingly, however, the α1a-adrenoceptor mRNA detected at the tissue level disappears in cultures of human prostatic smooth muscle cells, in contrast to the consistent expression of α1b- and α1d-adrenoceptor mRNAs (Boesch et al., 1999). The relative expression of α1a-adrenoceptor mRNA in the lower urinary tract of humans has also been examined by in situ hybridization studies. In the human prostate, α1a-adrenoceptor mRNA was mainly detected in the stroma, including smooth muscle cells, but not in the glandular epithelium (Walden et al., 1999).
Data regarding the expression of α1-adrenoceptor mRNA in the human bladder are inconsistent, but it appears that the expression of α1-adrenoceptor mRNA is extremely low in the bladder (Malloy et al., 1998; Nomiya and Yamaguchi, 2003). In the human detrusor, β (mainly β3)-adrenoceptors are dominant over α-adrenoceptors, based on the fact that the normal response to noradrenaline is relaxation rather than contraction (Andersson, 1993). Therefore, we can conclude that the functional significance of α-adrenoceptors in the detrusor contraction may be marginal or non-existent (Fig. 1). On the other hand, the density of noradrenergic nerves increases markedly towards the bladder neck, where the smooth muscle receives a dense noradrenergic nerve supply (Gosling et al., 1999). In the human bladder, the predominant expression of α1a-adrenoceptor mRNA in the dome, trigone and base has also been reported (Walden et al., 1997).
Thus, the mRNA data in human urinary tract indicate that the α1a-adrenoceptor polypeptide is a potential target for pharmacotherapy in patients with BPH and SUI.
α1-Adrenoceptor pharmacological anomalies in the lower urinary tract
The classical α1-adrenoceptors (α1A, α1B and α1D) show high affinity for prazosin (pKi or pKB > 9) in most binding and functional studies (Lomasney et al., 1991; Hieble et al., 1995; Ford et al., 1996; Theroux et al., 1996; Taniguchi et al., 1999; Suzuki et al., 2000; Israilova et al., 2004) (Table 2). However, the functional α1-adrenoceptors identified in the lower urinary tract are known to be relatively resistant to prazosin (Muramatsu et al., 2009) (pKB=∼8) (Table 1). Thus, this pharmacological anomaly, despite the predominance of α1a-adrenoceptor mRNA, caused confusion about the α1-adrenoceptor subtypes in the lower urinary tract. Because different affinities for antagonists have traditionally been regarded as characteristic pharmacological criteria for defining novel receptors (Kenakin, 1995; Rang, 2006), the anomalous α1-adrenoceptor showing low affinity for prazosin suggests the existence of a distinctive subtype (or phenotype).
Pharmacological heterogeneity of α1-adrenoceptors was originally reported by Drew (1985), who first noted a wide variation in the functional affinities for yohimbine and prazosin. This evidence was confirmed in experiments with isolated blood vessels by Flavahan and Vanhoutte (1986), and they proposed two distinct α1-adrenoceptor subtypes that could be distinguished by their different affinities for both prazosin and yohimbine (α1H and α1L according to their High or Low affinity for prazosin). This subclassification was subsequently extended by many pharmacologists and in many tissues including the lower urinary tract (Muramatsu et al., 1990; 1991; 1995; Ford et al., 1996; Testa et al., 1997; Stam et al., 1999; Argyle and McGrath, 2000). In this subclassification, the classical α1A-, α1B- and α1D-adrenoceptors are included in the α1H group, and the anomalous α1-adrenoceptor showing low affinity for prazosin in the lower urinary tract has been reported as the α1L-adrenoceptor (Muramatsu et al., 1991; 1995; Ford et al., 1996; Langer, 1999; Nishimune et al., 2010a). Antagonistic potencies against noradrenaline-induced contractions in rat, rabbit and human prostate are low for prazosin (pKB=∼8) and α1A-antagonists (5-methylurapidil, WB4101, RS-17053), but are high for silodosin and tamsulosin (Hiraoka et al., 1995; 1999; Ford et al., 1996; Leonardi et al., 1997; Testa et al., 1997; Van der Graaf et al., 1997; Morishima et al., 2007; Su et al., 2008). In contrast to this nomenclature, α1-adrenoceptors in the lower urinary tract have been also named as α1A-adrenoceptors in many reports (Table 1). Therefore, there has been confusion in defining the functional α1-adrenoceptor in the lower urinary tract (Table 1), which may in part depend on distinct subclassifications (α1H, α1L vs. α1A, α1B, α1D).
α2-Adrenoceptor can also be detected at the mRNA and protein levels in urogenital tissues (Yablonsky et al., 1986; Michel and Vrydag, 2006). Therefore, the anomalous characteristics of adrenergic contractions in the lower urinary tract may be associated with the α2-adrenoceptor. In fact, clonidine (α2-agonist) can produce a significant contraction in the female rabbit urethra (Larsson et al., 1986). However, clonidine is inactive in the isolated female human urethra, while noradrenaline produces contractions through sites with low affinity for prazosin (Taki et al., 1999). These results show that the α2-adrenoceptor is not significantly involved in the anomalous contraction in the human urethra (Ruffolo and Hieble, 1999), although there may be relevant species differences in this adrenoceptor.
Identification of α1-adrenoceptors by radioligand binding assay
Because reliable subtype-specific α1a-adrenoceptor antibodies were recently reported to be unavailable (Jensen et al., 2009), investigation of α1-adrenoceptors at the protein level are herein summarized from radioligand-binding experiments.
Binding assay with membrane homogenates
Specific binding of [3H]-prazosin, [125I]-HEAT, [3H]-tamsulosin and [3H]-silodosin to α1-adrenoceptors in membrane preparations of the lower urinary tract has been detected, with the density of binding in the order, prostate > urethra > bladder. In the rat, rabbit and human prostate, the most abundant α1-adrenoceptor is the α1A-subtype, which has been identified as having high affinity for prazosin, tamsulosin, silodosin and other α1A-selective antagonists (5-methylurapidil and RS-17053) (Tables 1 and 2). Thus, low-affinity sites for prazosin (α1L-adrenoceptors) are not detected in the membrane preparations of lower urinary tract tissues and at subnanomolar concentrations of [3H]-prazosin. The high abundance of the binding sites showing α1A-adrenoceptor profile in the lower urinary tract is in agreement with the mRNA expression data mentioned above.
Three α1-adrenoceptor cDNAs (including splice variants of α1A-adrenoceptors) were transfected in several cell lines (CHO cells, COS-7 cells, HEK293 cells, HeLa cells) and the pharmacological binding characteristics in the membrane preparations were compared with those in native tissues. The three recombinant α1-adrenoceptors showed high (subnanomolar) affinity for prazosin (Table 2). The cloned α1a-adrenoceptor and its splice variants showed the same pharmacological profile as that recognized in the membrane preparations of lower urinary tract (Lomasney et al., 1991; Theroux et al., 1996; Piao et al., 2000; Suzuki et al., 2000; Ramsay et al., 2004).
Binding assay with whole cells and intact tissues
Most radioligand-binding studies conducted to date have involved homogenates or membrane preparations of tissues or cells as a source of receptors. Because tissue/cell homogenization physically disturbs the receptor micro-environment, it may cause changes in some of the pharmacological characteristics of the receptor. Therefore, in order to retain the natural/native receptor conformation by minimizing physical agitation, whole cells or intact tissue segments must be used in binding assays.
Ford and co-workers reported that the pharmacological characteristics of the recombinant α1a-adrenoceptor can vary substantially depending upon assay conditions (Ford et al., 1997; Daniels et al., 1999). In CHO cells expressing the recombinant α1a-adrenoceptor, prazosin and RS-17053 (α1A-selective antagonist) show substantially lower potency against functional responses (phosphatidyl inositol turnover and calcium influx) in intact cells than inhibition of radioligand binding to α1a-adrenoceptors in membrane homogenates. Furthermore, the Ki values for prazosin and RS-17053 as competitive inhibitors of [3H]-prazosin binding to α1a-adrenoceptors in these CHO cells are dependent on assay conditions, with lower affinity observed when binding is conducted under more physiological conditions (culture medium, intact cells, 37°C) than under conditions commonly employed for radioligand binding assays (artificial buffer, membrane homogenates, 20°C). These observations have led to the proposal that the α1L-adrenoceptor may not represent an independent molecular entity, but rather may be an ‘affinity state’ of the α1A-adrenoceptor that is predominant in lower urinary tract.
Recently, a tissue segment binding method was developed and applied to numerous tissues (Tanaka et al., 2004; Muramatsu et al., 2005). In this method, tissue segments are incubated (without homogenization) in a nutrient medium such as Krebs solution during the course of ligand binding. To identify the α1-adrenoceptors having low affinity for prazosin, [3H]-silodosin was used instead of [3H]-prazosin, because [3H]-prazosin at subnanomolar concentrations cannot bind sufficiently to α1L-adrenoceptors (Su et al., 2008; Muramatsu et al., 2009). Silodosin and its tritiated radioligand are known to be of equally high affinity for both the α1A- and α1L-adrenoceptors (Murata et al., 1999; Su et al., 2008) (Table 2).
In segments of human, mouse and rabbit prostate, the binding of [3H]-silodosin was biphasically displaced by prazosin, indicating the coexistence of high and low-affinity sites for prazosin, which correspond to α1A- and α1L-adrenoceptors (Morishima et al., 2007; Muramatsu et al., 2008; Su et al., 2008). This result is very different from the results obtained in homogenates, in which a single high-affinity site for prazosin (α1A-adrenoceptor) was detected. The low-affinity site for prazosin in the prostate segments also showed low affinity for some α1A-selective antagonists (RS-17053, 5-methylurapidil), but tamsulosin (which has high affinity for α1A-, α1L- and α1D-subtypes) did not discriminate either the high- or low-affinity sites for prazosin. Similar results were obtained in studies of other tissues in which the α1L-adrenoceptor was identified as a functional receptor (Hiraizumi-Hiraoka et al., 2004; Morishima et al., 2008; Muramatsu et al., 2008; 2009). From these observations, it was suggested that α1L-adrenoceptors (or α1L-phenotype) coexist with α1A-adrenoceptors (or α1A-phenotype) as pharmacologically distinct entities under intact segment conditions, whereas the pharmacological profile of α1L-adrenoceptors converts to the α1A-phenotype upon homogenization.
More recently, this conclusion was again confirmed in a recombinant system (Nishimune et al., 2010b). In the CHO cell line, transfection of α1a-adrenoceptor cDNA predominantly expresses α1A-adrenoceptor phenotype, with an extremely minor proportion of α1L-adrenoceptor (less than 10% of total α1-adrenoceptor). However, persistent over-expression of the protein, cysteine-rich epidermal growth factor-like domain 1α (CRELD1α), which was found as a potential α1a-adrenoceptor-interacting protein candidate) strongly reduced the population of α1A-adrenoceptor phenotype in CHO cells (Nishimune et al., 2010b). Although mechanisms underlying the interactions between generation of CRELD1α and α1A-adrenoceptors and α1L-adrenoceptors remain unclear, two distinct (α1L-adrenoceptor-dominant and α1A-adrenoceptor-dominant) CHO cell lines were eventually established. Under whole-cell conditions, in contrast to the α1A-adrenoceptor, pharmacological and functional properties of the established α1L-adrenoceptor show low affinity for prazosin and other α1A-adrenoceptor antagonists (5-methylurapidil, RS-17053), and the agonist and antagonist pharmacology is consistent with the profile of the α1L-adrenoceptor identified in the lower urinary tract. Therefore, from these lines of evidence, it may be now concluded that the α1L-adrenoceptor is one of the α1a-adrenoceptor gene products and occurs as an entity distinct from the α1A-adrenoceptor phenotype under conditions when the tissue/cell is kept intact. This conclusion would also explain why α1L-adrenoceptors could not be detected after homogenization but are easily recognized in functional bioassay studies with intact tissue strips (Muramatsu et al., 2009).
Identification of the gene encoding α1L-adrenoceptor
Despite extensive searches at early stages after the proposal of the α1L-adrenoceptor and the subsequent completion of the human genome sequencing project, a distinct gene for the proposed α1L-adrenoceptor has not been identified. Rather, as described above, a close relationship between α1L-adrenoceptor and α1A-adrenoceptor has been considered (Ford et al., 1997; Hiraizumi-Hiraoka et al., 2004; Morishima et al., 2008).
In order to explore this possible link between α1A- and α1L-adrenoceptors, we analysed in vivo phenotypes of mice having disrupted alleles of the classical α1-adrenoceptors (α1A, α1B and α1D) (Muramatsu et al., 2008). The integrity of the α1L-adrenoceptors were confirmed both in terms of ligand binding properties and contractile function in the Adra1B−/− (α1b−/−) and Adra1D−/− (α1d−/−) mice as well as in the wild-type mice. In contrast, both α1L- and α1A-adrenoceptors were completely absent from the Adra1A−/− (α1a−/−) mice. These results unequivocally demonstrate that both α1A-adrenoceptors and α1L-adrenoceptors are derived from the same Adra1A (α1a) gene (Gray et al., 2008; Muramatsu et al., 2008) (Table 2).
Observation of α1L-adrenoceptor distribution by fluorescent probe
Alexa Fluor 488 dye conjugated with silodosin (Alexa-488-silodosin) was recently introduced as a fluorescent probe (Morishima et al., 2010). Alexa-488-silodosin retains the high affinity and selectivity for α1A- and α1L-adrenoceptors as shown by unlabelled silodosin. Histochemical experiments with this fluorescent probe clearly showed that Alexa-488-silodosin binds to the smooth muscle but not the glandular tissue in the human prostate, and that the binding is resistant to low concentrations of prazosin. These results are in good agreement with in situ hybridization data showing selective expression of α1A-adrenoceptor mRNA in the stroma of the human prostate (Walden et al., 1999). As the Alexa-488-silodosin can specifically label α1L-adrenoceptors, particularly by co-incubation with low concentrations of prazosin (to mask the α1A-adrenoceptor), this novel molecular probe provides a versatile tool to study α1L-adrenoceptor histochemically (Morishima et al., 2010).
Perspective
As mentioned above, recent progress in this field clearly demonstrates that two distinct α1-adrenoceptor phenotypes (α1A and α1L) originate from a single α1a-adrenoceptor gene and coexist in some tissues. However, adrenergic contraction in the lower urinary tract is predominantly mediated through α1L-, but not α1A-adrenoceptors (Fig. 1). In general, the affinity values (pKi or pKB) for prazosin for α1L-adrenoceptors are around 8, but recent studies reveal a further variation in prazosin affinity (pKi or pKB= 6.3–8.5) at various α1L-adrenoceptors in many tissues and species (Muramatsu et al., 2009). This is reminiscent of the original question on α1-adrenoceptors (Drew, 1985), suggesting further heterogeneity in α1L-adrenoceptor pharmacology. At present, the mechanisms underlying the expression of α1L-adrenoceptor phenotype and its functional predominance in several tissues remain unknown. However, it is likely that the expression of divergent α1L-adrenoceptor phenotypes is strongly dependent on any modification of the tissues of various species, rather than a simple variation of the α1a-adrenoceptor protein or additional subtypes (Muramatsu et al., 2009; Nishimune et al., 2010a).
Recently, ample evidence has been accumulating suggesting that antagonist affinity is not necessarily constant at a given receptor expressed in different tissues/cells and examined under different assay conditions (Kenakin, 2003; Baker and Hill, 2007; Nelson and Challiss, 2007; Muramatsu et al., 2008). The α1L-adrenoceptor exemplifies this type of variable affinity, as after homogenization, the phenotype changed from α1L into α1A. It is likely that tissue integrity is an important factor to determine receptor properties (Su et al., 2008; Muramatsu et al., 2009). Therefore, we may have to re-evaluate pharmacodynamic and pharmacokinetic effects of currently used drugs, and to reconstruct drug development strategies. Now, α1-antagonists, such as tamsulosin, silodosin, alfuzosin, are clinically used in BPH patients. According to the evidence mentioned above, these antagonists appear to act mainly on functional α1L-adrenoceptors in the lower urinary tract, but are not specific for the α1L-adrenoceptor. The α1L-adrenoceptors in the female urethra may be a new target in therapy for SUI. For this purpose, two α1-adrenoceptor agonists (NS-49 and Ro 115-1240) have been developed. However, these compounds are full agonists of the α1A-adrenoceptor or partial agonists of the α1A/L-adrenoceptor (Obika et al., 1995; Blue et al., 2004; Musselman et al., 2004). Thus, more selective or specific drugs against α1L-adrenoceptors may lead to improved uroselectivity.
Conclusion
Since the successful cloning of most receptors, it has become possible to elucidate numerous physiological responses by genome-based subtype (genotypes). However, there are still some unique phenotypes showing distinct pharmacology in native tissues. The α1L-adrenoceptor is representative of this group, and originates from the α1a-adrenoceptor gene together with the α1A-adrenoceptor phenotype. In this review, we propose that different phenotypes are expressed from a single gene in native tissues (‘one gene-multiple phenotypes theory’), which may explain the long controversy regarding some putative receptors, such as α1L-adrenoceptor in the lower urinary tract, and further highlights phenotype-dependent pharmacology (‘phenotype pharmacology’).
Acknowledgments
This work was supported in part by a Grant-in Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS); by the Adaptable and Seamless Technology Transfer Program through target-driven R&D from the Japan Science and Technology Agency (JST); and by a grant from the Smoking Research Foundation of Japan. Anisuzzaman ASM was supported by the Headquarters for the Advancement of High Priority Research Grant, Fukui University.
Glossary
- BPH
benign prostatic hyperplasia
- CRELD1α
cycteine-rich epidermal growth factor-like domain 1α
- NS-49
(R)-(-)-3′-(2-amino-1-hydroxyethyl)-4′-fluoromethanesulphonanilide hydrochloride
- Ro
115-1240 (Dabuzalgron, R-450), N-[6-chloro-3-(4,5-dihydro-1H-imidazol-2-ylmethoxy)-2-methylphenyl] methanesulphonamide
- RS-17053
N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α, α-dimethyl-1 H-indole-3-ethanamine hydrochloride
- silodosin
1-(3-hydroxypropyl)-5-[(2R)-({2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino) propyl] indoline-7-carboxamide
- SUI
stress urinary incontinence
- WB4101
2(N[2,6-dimethyoxyphenoxyethyl])amino-methyl-1,4-benzodioxane
Conflict of interest
The authors declare no competing financial interests.
References
- Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- Andersson KE. Alpha-adrenoceptors and benign prostatic hyperplasia: basic principles for treatment with alpha-adrenoceptor antagonists. World J Urol. 2002;19:390–396. [PubMed] [Google Scholar]
- Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- Argyle SA, McGrath JC. An α1A/ α1L-adrenoceptor mediates contraction of canine subcutaneous resistance arteries. J Pharmacol Exp Ther. 2000;295:627–633. [PubMed] [Google Scholar]
- Baker JG, Hill SJ. Multiple GPCR conformations and signalling pathways: implications for antagonist affinity estimates. Trends Pharmacol Sci. 2007;28:374–381. doi: 10.1016/j.tips.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue DR, Daniels DV, Gever JR, Jett MF, O'yang C, Tang HM, et al. Pharmacological characteristics of Ro 115–1240, a selective α1A/1L-adrenoceptor agonist: a potential therapy of stress urinary incontinence. BJU Int. 2004;93:162–170. doi: 10.1111/j.1464-410x.2004.04577.x. [DOI] [PubMed] [Google Scholar]
- Boesch ST, Corvin S, Zhang J, Rogatsch H, Bartsch G, Klocker H. Modulation of the differentiation status of cultured prostatic smooth muscle cells by an α1-adrenergic receptor antagonist. Prostate. 1999;39:226–233. doi: 10.1002/(sici)1097-0045(19990601)39:4<226::aid-pros2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Chang RS, Chen TB, O'malley SS, Pettibone DJ, Disalvo J, Francis B, et al. In vitro studies on L-771, 688 (SNAP 6383), a new potent and selective α1A-adrenoceptor antagonist. Eur J Pharmacol. 2000;409:301–312. doi: 10.1016/s0014-2999(00)00854-2. [DOI] [PubMed] [Google Scholar]
- Chapple CR. Alpha adrenoceptor antagonists in the year 2000: is there anything new? Curr Opin Urol. 2001;11:9–16. doi: 10.1097/00042307-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R, Chapple CR, Verfurth F, Noble AJ, Couldwell CJ, Michel MC. The effects of SB 216469, an antagonist which discriminates between the α1Aadrenoceptor and the human prostatic α1-adrenoceptor. Br J Pharmacol. 1996;119:1093–1100. doi: 10.1111/j.1476-5381.1996.tb16009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, McKiernan JM, Kaplan SA. Alpha-adrenoceptor antagonists in the treatment of benign prostatic hyperplasia. Drugs. 1999;57:9–17. doi: 10.2165/00003495-199957010-00002. [DOI] [PubMed] [Google Scholar]
- Daniels DV, Gever JR, Jasper JR, Kava MS, Lesnick JD, Meloy TD, et al. Human cloned alpha1A-adrenoceptor isoforms display alpha1L-adrenoceptor pharmacology in functional studies. Eur J Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- Delaflotte S, Auguet M, Chabrier PE. Pharmacological evidence that different α1 adrenoceptor subtypes mediate contraction in rabbit prostate and hypogastric artery. Acta Physiol Scand. 1996;158:241–251. doi: 10.1046/j.1365-201X.1996.565310000.x. [DOI] [PubMed] [Google Scholar]
- Deplanne V, Galzin AM. Functional characterization of alpha-1-adrenoceptor subtypes in the prostatic urethra and trigone of male rabbit. J Pharmacol Exp Ther. 1996;278:527–534. [PubMed] [Google Scholar]
- Drew GM. What do antagonists tell us about α1-adrenoceptors? Clin Sci. 1985;68(Suppl. 10):15s–19s. doi: 10.1042/cs068s015. [DOI] [PubMed] [Google Scholar]
- Eltze M, Bper R, Michel MC, Hein P, Testa R, Ulrich WR, et al. In vitro and in vivo uroselectivity of B8805–033, an antagonist with high affinity at prostatic α1A- vs α1B- and α1D-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:649–662. doi: 10.1007/s002100100413. [DOI] [PubMed] [Google Scholar]
- Faure C, Pimoule C, Vallancien G, Langer SZ, Graham D. Identification of α1-adrenoceptor subtypes present in the human prostate. Life Sci. 1994;54:1595–1605. doi: 10.1016/0024-3205(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Vanhoutte PM. α1-Adrenoceptor subclassification in vascular smooth muscle. Trends Pharmacol Sci. 1986;7:347–349. [Google Scholar]
- Ford AP, Arredondo NF, Blue DR, Jr, Bonhaus DW, Jasper J, Kava MS, et al. RS-17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-alpha, alpha-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective alpha 1A-adrenoceptor antagonist, displays low affinity for functional alpha 1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray C, Bard JA, Wetzel JM, Chiu G, Shapiro E, Tang R, et al. The α1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned human α1C subtype. Mol Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- Foster HE, Jr, Yono M, Shin D, Takahashi W, Pouresmail M, Afiatpour P, et al. Effects of chronic administration of doxazosin on α1-adrenoceptors in the rat prostate. J Urol. 2004;172:2465–2470. doi: 10.1097/01.ju.0000138475.89790.88. [DOI] [PubMed] [Google Scholar]
- Gosling JA, Dixon JS, Jen PYP. The distribution of noradrenergic nerves in the human lower urinary tract. Eur Urol. 1999;38(Suppl. 1):23–30. doi: 10.1159/000052314. [DOI] [PubMed] [Google Scholar]
- Gray K, Short J, Ventura S. The alpha1A-adrenoceptor gene is required for the alpha1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate. Br J Pharmacol. 2008;155:103–109. doi: 10.1038/bjp.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel C, Dolber PC, Smith MP, Savic DL, Thuroff JW, Thor KB, et al. Modulation of bladder α1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002;167:1513–1521. [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Hiraizumi-Hiraoka Y, Tanaka T, Yamamoto H, Suzuki F, Muramatsu I. Identification of alpha-1L adrenoceptor in rabbit ear artery. J Pharmacol Exp Ther. 2004;310:995–1002. doi: 10.1124/jpet.104.066985. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Ohmura T, Sakamoto S, Hayashi H, Muramatsu I. Identification of α1-adrenoceptor subtypes in the rabbit prostate. J Auton Pharmacol. 1995;15:271–278. doi: 10.1111/j.1474-8673.1995.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Ohmura T, Oshita M, Watanabe Y, Morikawa K, Nagata O, et al. Binding and functional characterization of α1-adrenoceptor subtypes in the rat prostate. Eur J Pharmacol. 1999;366:119–126. doi: 10.1016/s0014-2999(98)00895-4. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Horie K, Tanaka T, Takagaki K, Murai M, Yano J, et al. Cloning, functional expression and tissue distribution of human cDNA for the α1C-adrenergic receptor. Biochem Biophys Res Commun. 1993;195:902–909. doi: 10.1006/bbrc.1993.2130. [DOI] [PubMed] [Google Scholar]
- Homma Y, Hamada K, Nakayama Y, Tsujimoto G, Kawabe K. Effect of castration on contraction and α1-adrenoceptor expression in rat prostate. Br J Pharmacol. 2000;131:1454–1460. doi: 10.1038/sj.bjp.0703706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Nakagawa C. Alpha-1 adrenoceptor antagonist effects of the optical isomers of YM-12617 in rabbit lower urinary tract and prostate. J Pharmacol Exp Ther. 1986;239:512–516. [PubMed] [Google Scholar]
- Honda K, Miyata-Osawa A, Takenaka T. α1-Adrenoceptor subtype mediating contraction of the smooth muscle in the lower urinary tract and prostate of rabbits. Naunyn Schmiedeberg Arch Pharmacol. 1985;330:16–21. doi: 10.1007/BF00586704. [DOI] [PubMed] [Google Scholar]
- Israilova M, Tanaka T, Suzuki F, Morishima S, Muramatsu I. Pharmacological characterization and cross talk of α1A- and α1B-adrenoceptors coexpressed in human embryonic kidney 293 cells. J Pharmacol Exp Ther. 2004;309:259–266. doi: 10.1124/jpet.103.061796. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedeberg Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kava MS, Blue DR, Jr, Vimont RL, Clarke DE, Ford AP. α1L-Adrenoceptor mediation of smooth muscle contraction in rabbit bladder neck: a model for lower urinary tract tissues of man. Br J Pharmacol. 1998;123:1359–1366. doi: 10.1038/sj.bjp.0701748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. On the importance of the ‘antagonist assumption’ to how receptors express themselves. Biochem Pharmacol. 1995;50:17–26. doi: 10.1016/0006-2952(95)00137-o. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Predicting therapeutic value in the lead optimization phase of drug discovery. Nat Rev Drug Discov. 2003;2:429–438. doi: 10.1038/nrd1110. [DOI] [PubMed] [Google Scholar]
- Lagu B, Tian D, Jeon Y, Li C, Wetzel JM, Nagarathnam D, et al. De novo design of a novel oxazolidinone analogue as a potent and selective α1A adrenergic receptor antagonist with high oral bioavailability. J Med Chem. 2000;43:2775–2778. doi: 10.1021/jm000085e. [DOI] [PubMed] [Google Scholar]
- Langer SZ. History and nomenclature of α1-adrenoceptors. Eur Urol. 1999;36(Suppl. 1):2–6. [PubMed] [Google Scholar]
- Larsson B, Sjogren C, Andersson KE. Regional distribution of alpha-adrenoceptor subtypes in the female rabbit urethra. Acta Physiol Scand. 1986;126:39–43. doi: 10.1111/j.1748-1716.1986.tb07786.x. [DOI] [PubMed] [Google Scholar]
- Lefevre-Borg F, O'Connor SE, Schoemaker H, Lechaire J, Gautier E, et al. Alfuzosin, a selective alpha 1-adrenoceptor antagonist in the lower urinary tract. Br J Pharmacol. 1993;109:1282–1289. doi: 10.1111/j.1476-5381.1993.tb13762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A, Hieble JP, Guarneri L, Naselsky DP, Poggesi E, Sironi G, et al. Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part I. J Pharmacol Exp Ther. 1997;281:1272–1283. [PubMed] [Google Scholar]
- Levin RM, Ruggieri MR, Wein AJ. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J Urol. 1988;139:844–848. doi: 10.1016/s0022-5347(17)42659-0. [DOI] [PubMed] [Google Scholar]
- Lluel P, Palea S, Ribière P, Barras M, Teillet L, Corman B. Increased adrenergic contractility and decreased mRNA expression of NOS III in aging rat urinary bladders. Fundam Clin Pharmacol. 2003;17:633–641. doi: 10.1046/j.1472-8206.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- Lomasney JW, Cotecchia S, Lefkowitz RJ, Caron MG. Molecular biology of alpha-adrenergic receptors: implications for receptor classification and for structure-function relationships. Biochim Biophys Acta. 1991;1095:127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Malloy BJ, Price DT, Price RR, Bienstock AM, Dole MK, Funk BL, et al. α1-Adrenergic receptor subtypes in human detrusor. J Urol. 1998;160:937–943. doi: 10.1016/S0022-5347(01)62836-2. [DOI] [PubMed] [Google Scholar]
- Marshall I, Burt RP, Chapple CR. Noradrenaline contractions of human prostate mediated by α1A- (α1c-) adrenoceptor subtype. Br J Pharmacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Vrydag W. α1-, α2- And β-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147:S88–S119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Grubbel B, Taguchi K, Verfurth F, Otto T, Kropfl D. Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned α1-adrenoceptor subtypes and in human prostate. J Auton Pharmacol. 1996;16:21–28. doi: 10.1111/j.1474-8673.1996.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Michelotti GA, Price DT, Schwinn DA. Alpha-1 adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- Morishima S, Tanaka T, Yamamoto H, Suzuki F, Akino H, Yokoyama O, et al. Identification of alpha-1L and alpha-1A adrenoceptors in human prostate by tissue segment binding. J Urol. 2007;177:377–381. doi: 10.1016/j.juro.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Morishima S, Suzuki F, Yoshiki H, Anisuzzaman ASM, Sathi ZS, Tanaka T, et al. Identification of alpha-1L adrenoceptor in rat cerebral cortex and possible relationship between alpha-1L and alpha-1A adrenoceptors. Br J Pharmacol. 2008;153:1485–1494. doi: 10.1038/sj.bjp.0707679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima S, Suzuki F, Nishimune A, Yoshiki H, Akino H, Yokoyama O, et al. Visualization and tissue distribution of α1L-adrenoceptor in human prostate by the fluorescently labeled ligand alexa-488-silodosin. J Urol. 2010;183:812–819. doi: 10.1016/j.juro.2009.09.078. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Ohmura T, Kigoshi S, Hashimoto S, Oshita M. Pharmacological subclassification of alpha 1-adrenoceptors in vascular smooth muscle. Br J Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I, Kigoshi S, Ohmura T. Subtypes of α1-adrenoceptors involved in noradrenaline-induced contractions of rat thoracic aorta and dog carotid artery. Jpn J Pharmacol. 1991;57:535–544. doi: 10.1254/jjp.57.535. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Ohmura T, Hashimoto S, Oshita M. Functional subclassification of vascular α1-adrenoceptors. Pharmacol Commun. 1995;6:23–28. [Google Scholar]
- Muramatsu I, Tanaka T, Suzuki F, Li Z, Hiraizumi-Hiraoka Y, Anisuzzaman AS, et al. Quantifying receptor properties: the tissue segment binding method – a powerful tool for the pharmacome analysis of native receptors. J Pharmacol Sci. 2005;98:331–339. doi: 10.1254/jphs.cpj05001x. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Morishima S, Suzuki F, Yoshiki H, Anisuzzaman AS, Tanaka T, et al. Identification of alpha 1L-adrenoceptor in mice and its abolition by alpha 1A-adrenoceptor gene knockout. Br J Pharmacol. 2008;155:1224–1234. doi: 10.1038/bjp.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I, Suzuki F, Nishimune A, Anisuzzaman AS, Yoshiki H, Su TH, et al. Expression of distinct α1-adrenoceptor phenotypes in pigmented and albino rabbit iris. Br J Pharmacol. 2009;158:354–360. doi: 10.1111/j.1476-5381.2009.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Taniguchi T, Muramatsu I. Pharmacological analysis of the novel, selective alpha1-adrenoceptor antagonist, KMD-3213, and its suitability as a tritiated radioligand. Br J Pharmacol. 1999;127:19–26. doi: 10.1038/sj.bjp.0702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DM, Ford AP, Gennevois DJ, Harvison ML, Laurent AL, Mokatrin AS, et al. A randomized crossover study to evaluate Ro 115–1240, a selective a1A/L-adrenoceptor partial agonist in women with stress urinary incontinence. BJU Int. 2004;93:78–83. doi: 10.1111/j.1464-410x.2004.04560.x. [DOI] [PubMed] [Google Scholar]
- Nasu K, Moriyama N, Kawabe K, Tsujimoto G, Murai M, Tanaka T, et al. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br J Pharmacol. 1996;119:797–803. doi: 10.1111/j.1476-5381.1996.tb15742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu K, Moriyama N, Fukasawa R, Tsujimoto G, Tanaka T, Yano J, et al. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human proximal urethra. Br J Pharmacol. 1998;123:1289–1293. doi: 10.1038/sj.bjp.0701731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CP, Challiss RA. ‘Phenotypic’ pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem Pharmacol. 2007;73:737–751. doi: 10.1016/j.bcp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Suzuki F, Yoshiki H, Morishima S, Muramatsu I. α1-Adrenoceptor pharmacome: α1L-adrenoceptor and α1?-adrenoceptor in the lower urinary tract. Int J Urol. 2010a;17:31–37. doi: 10.1111/j.1442-2042.2009.02368.x. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Suzuki F, Yoshiki H, Morishima S, Muramatsu I. Identification of Cysteine-rich epidermal growth factor-like domain 1α (CRELD1α) as a novel α1?-adrenoceptor-down-regulating protein and establishment of an α1L-adrenoceptor-expressing cell line. J Pharmacol Sci. 2010b;113:169–181. doi: 10.1254/jphs.10093fp. [DOI] [PubMed] [Google Scholar]
- Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of α1 and β-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- Obika A, Shibata A, Horie K, Foglar R, Kimura K, Tsuijimoto G. NS-49, a novel α1a-adrenoceptor-selective agonist using recombinant human α1-adrenoceptors. Eur J Pharmacol. 1995;291:327–334. doi: 10.1016/0922-4106(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Piao H, Taniguchi T, Nakamura S, Zhu J, Suzuki F, Mikami D, et al. Cloning of rabbit α1b-adrenoceptor and pharmacological comparison of α1a-, α1b- and α1d-adrenoceptors in rabbit. Eur J Pharmacol. 2000;396:9–17. doi: 10.1016/s0014-2999(00)00171-0. [DOI] [PubMed] [Google Scholar]
- Price DT, Schwinn DA, Lomasney JW, Allen LF, Caron MG, Lefkowitz RJ. Identification, quantification, and localization of mRNA for three distinct alpha1 adrenergic receptor subtypes in human prostate. J Urol. 1993;150:546–551. doi: 10.1016/s0022-5347(17)35544-1. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, et al. High-affinity interactions between human alpha1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the alpha1L-adrenoceptor. Mol Pharmacol. 2004;66:228–239. doi: 10.1124/mol.66.2.228. [DOI] [PubMed] [Google Scholar]
- Rang HP. The receptor concept: pharmacology's big idea. Br J Pharmacol. 2006;147:S9–S16. doi: 10.1038/sj.bjp.0706457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokosh DG, Bailey BA, Stewart AF, Karns LR, Long CS, Simpson PC. Distribution of α1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with α1B and α1D. Biochem Biophys Res Commun. 1994;200:1177–1184. doi: 10.1006/bbrc.1994.1575. [DOI] [PubMed] [Google Scholar]
- Ruffolo R, Jr, Hieble JP. Adrenoceptor Pharmacology: urogenital applications. Eur Urol. 1999;36(Suppl. 1):17–22. doi: 10.1159/000052313. [DOI] [PubMed] [Google Scholar]
- Scofield MA, Liu F, Abel PW, Jeffries WB. Quantification of steady state expression of mRNA for alpha-1 adrenergic receptor subtypes using reverse transcription and a competitive polymerase chain reaction. J Pharmacol Exp Ther. 1995;275:1035–1042. [PubMed] [Google Scholar]
- Stam WB, Van der Graaf PH, Saxena PR. Analysis of alpha 1L-adrenoceptor pharmacology in rat small mesenteric artery. Br J Pharmacol. 1999;127:661–670. doi: 10.1038/sj.bjp.0702598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TH, Morishima S, Suzuki F, Yoshiki H, Anisuzzaman AS, Tanaka T, et al. Native profiles of α1A-adrenoceptor phenotypes in rabbit prostate. Br J Pharmacol. 2008;155:906–912. doi: 10.1038/bjp.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Taniguchi T, Takauji R, Murata S. Splice isoforms of α1a-adrenoceptor in rabbit. Br J Pharmacol. 2000;129:1569–1576. doi: 10.1038/sj.bjp.0703242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Hatano A, Arai K, Obara K, Tsutsui T, Takahashi K. Alpha1- and alpha 2-adrenoceptors in BPH. Eur Urol. 1999;36(Suppl. 1):31–34. doi: 10.1159/000052315. [DOI] [PubMed] [Google Scholar]
- Taki N, Taniguchi T, Okada K, Moriyama N, Muramatsu I. Evidence for predominant mediation of α1-adrenoceptor in the tonus of entire urethra of women. J Urol. 1999;162:1829–2832. [PubMed] [Google Scholar]
- Tanaka T, Zhang L, Suzuki F, Muramatsu I. Alpha-1 adrenoceptors: evaluation of receptor subtype-binding kinetics in intact arterial tissues and comparison with membrane binding. Br J Pharmacol. 2004;141:468–476. doi: 10.1038/sj.bjp.0705627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Inagaki R, Murata S, Akiba I, Muramatsu I. Microphysiometric analysis of human α1a-adrenoceptor expressed in Chinese hamster ovary cells. Br J Pharmacol. 1999;127:962–968. doi: 10.1038/sj.bjp.0702609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Ibba M, Strada G, Poggesi E, Taddei C, et al. Characterization of α1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur J Pharmacol. 1993;249:307–315. doi: 10.1016/0014-2999(93)90527-o. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Angelico P, Poggesi E, Taddei C, Sironi G, et al. Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part II. J Pharmacol Exp Ther. 1997;281:1284–1293. [PubMed] [Google Scholar]
- Theroux TL, Esbenshade TA, Minneman KP. Coupling efficacies of human alpha 1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol Pharmacol. 1996;50:1376–1387. [PubMed] [Google Scholar]
- Tseng-Crank J, Kost T, Goetz A, Hazum S, Roberson KM, Haizlip J, et al. The α1C-adrenoceptor in human prostate: cloning, functional expression, and localization to specific prostatic cell types. Br J Pharmacol. 1995;115:1475–1485. doi: 10.1111/j.1476-5381.1995.tb16640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Graaf PH, Deplanne V, Duquenne C, Angel I. Analysis of α1-adrenoceptors in rabbit lower urinary tract and mesenteric artery. Eur J Pharmacol. 1997;327:25–32. doi: 10.1016/s0014-2999(97)89674-4. [DOI] [PubMed] [Google Scholar]
- Walden PD, Durkin MM, Lepor H, Wetzel JM, Gluchowski C, Gustafson EL. Localization of mRNA and receptor binding sites for the alpha 1a-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate. J Urol. 1997;157:1032–1038. [PubMed] [Google Scholar]
- Walden PD, Gerardi C, Lepor H. Localization and expression of the alpha1A-1, alpha1B and alpha1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J Urol. 1999;161:635–640. [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Geber JR, et al. In vitro α1-adrenoceptor pharmacology of Ro 70.0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br J Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonsky F, Riffaund JP, Lacolle JY, Dausse JP. Alpha 1- and alpha 2-adrenoceptors in the smooth muscle of male and female rabbit urethra. Eur J Pharmacol. 1986;11:1–8. doi: 10.1016/0014-2999(86)90385-7. [DOI] [PubMed] [Google Scholar]
- Yamagishi R, Akiyama K, Nakamura S, Hora M, Masuda N, Matsuzawa A, et al. Effect of KMD-3213, an α1a-adrenoceptor-selective antagonist, on the contractions of rabbit prostate and rabbit and rat aorta. Eur J Pharmacol. 1996;315:73–79. doi: 10.1016/s0014-2999(96)00589-4. [DOI] [PubMed] [Google Scholar]
- Yazawa H, Honda K. α1-Adrenoceptor subtype in the rat prostate is preferentially the α1A type. Jpn J Pharmacol. 1993;62:297–304. doi: 10.1254/jjp.62.297. [DOI] [PubMed] [Google Scholar]
- Yono M, Foster HE, Jr, Takahashi W, Pouresmail M, Latifpour J. Doxazosin-induced up-regulation of α1A−adrenoceptor mRNA in the rat lower urinary tract. Can J Physiol Pharmacol. 2004;82:872–878. doi: 10.1139/y04-098. [DOI] [PubMed] [Google Scholar]


