Figure 8.
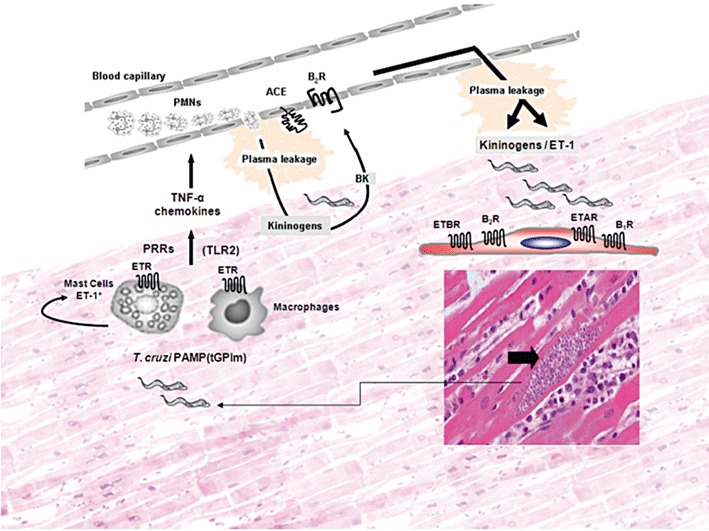
Interstitial oedema elicited by extracellular trypomastigotes might offer a window of opportunity for infection of heart cells via the endothelin/kinin pathways. Despite the scanty presence of parasitized heart cells in the myocardium of chronically infected Chagasic patients, we may predict that these pseudocysts occasionally burst (right/bottom side of panel), releasing high numbers of pro-inflammatory trypomastigotes into the surrounding interstitial spaces. On the left side of the panel, innate sentinel cells, such as macrophages and endothelin-positive mast cells, initiate inflammation through the sensing of microbial TLR2 ligands shed by extracellular TCTs. Following TLR2 signalling, activated mast cells may release ET-1, which then amplifies inflammation in an autocrine manner through ETR-driven secretion of histamine and TNF-α. Acting synergistically with CXC chemokines, these vasoactive mediators activate CXCR2 expressed by neutrophils/endothelium (Schmitz et al., 2009). We hypothesize that the ETR/B2R-driven leakage of plasma increases the concentrations of blood-borne kininogens and ET-1 (present at high levels in the serum of Chagasic patients) in the trypomastigote-laden infection sites (upper side of panel). T. cruzi trypomastigotes may then exploit this window of opportunity to invade cardiovascular cells via interdependent activation of ETRs and B2R and/or engaging the inducible B1R pathway (Todorov et al., 2003). The extracellular parasites may also take advantage of the up-regulated activation of ET-1/ETR pathway in parasite-infected cardiomyocytes (Petkova et al., 2000) to persistently infect cardiomyocytes in chronic Chagasic patients.
