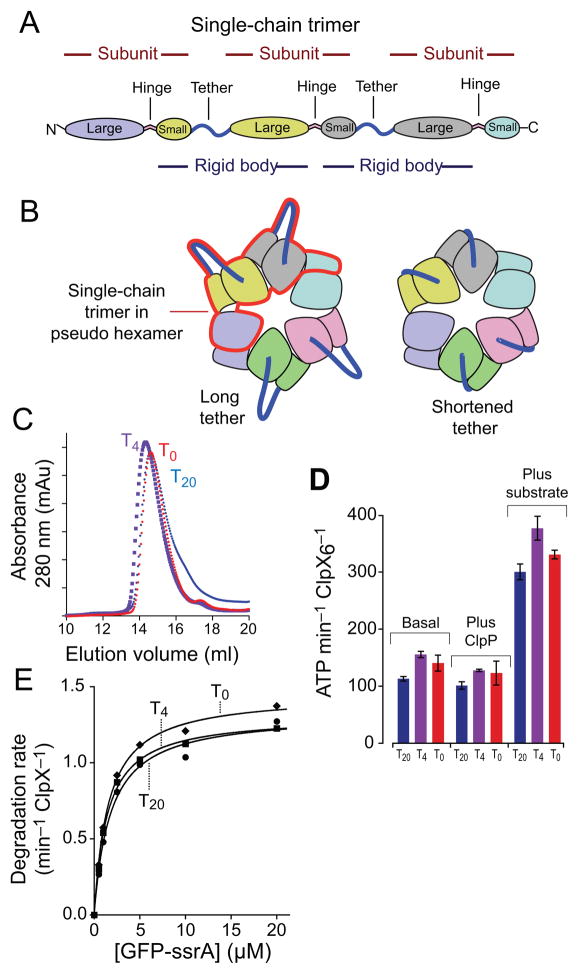
Figure 2.
Fusing rigid-body units with tethers of different lengths. (a) A single-chain ClpXΔN trimer contains three subunits and two polypeptide linkers that tether adjacent subunits. Each trimer contains two rigid-body units, which are fused by the tethers. (b) The position of one single-chain trimer in a pseudo hexamer is marked by the red outline. Shortening the tethers constrains potential movements of the large and small AAA+ domains of the rigid-body unit. (c) Single-chain ClpXΔN trimers with tethers of 20 residues (T20), 4 residues (T4), or zero residues (T0) chromatographed at similar positions on a Superose-6 gel-filtration column (void volume ~8 mL; GE Healthcare). A previous study15 showed that the T20 variant chromatographed identically to non tethered ClpXΔN at the position expected for a pseudo hexamer. (d) Pseudo hexamers formed from T0, T4, or T20 single-chain trimers catalyzed similar basal levels of ATP hydrolysis. This activity was repressed slightly by addition of ClpP and was stimulated markedly by addition of an unfolded protein substrate (carboxymethylated-titin-ssrA)20. (e) Pseudo hexamers (0.3 μM) formed from T0, T4, or T20 single-chain ClpXΔN trimers supported very similar levels of ClpP (0.9 μM) degradation of GFP-ssrA over a wide range of substrate concentrations. Symbols represent the average of three independent measurements. Lines are non-linear least-squares fits to the Michaelis-Menten equation. Values of KM and Vmax are listed in Table 1.