Abstract
The classical second messenger cAMP is important in diverse physiological processes, where its spatial and temporal compartmentalization allows precise control over multiple cellular events. Within this context, G-protein-coupled receptors (GPCRs) govern specialized pools of cAMP, which are functionally specific for the unique cellular effects attributed to a particular system. The relaxin receptor, RXFP1, is a GPCR that exerts pleiotropic physiological effects including a potent anti-fibrotic response, increased cancer metastases, and has efficacy as a vasodilator in heart failure. On a cellular level, relaxin stimulation of RXFP1 results in the activation of multiple G-protein pathways affecting cAMP accumulation. Specificity and diversity in the cAMP signal generated by RXFP1 is controlled by differential G-protein coupling dependent upon the background of cellular expression, and cAMP compartmentalization. Further complexity in cAMP signalling results from the constitutive assembly of an RXFP1–signalosome, which specifically responds to low concentrations of relaxin, and activates a distinct cAMP pathway. The RXFP1–signalosome is a higher-order protein complex that facilitates receptor sensitivity to attomolar concentration of peptide, exhibits constitutive activity and dual coupling to G-proteins and β-arrestins and reveals a concentration-biased agonism mediated by relaxin. The specific and directed formation of GPCR-centered signalosomes allows an even greater spatial and temporal control of cAMP, thus rationalizing the considerable physiological scope of this ubiquitous second messenger.
LINKED ARTICLES
This article is part of a themed section on the Molecular Pharmacology of G Protein-Coupled Receptors (GPCRs). To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-6. To view the 2010 themed section on the same topic visit http://onlinelibrary.wiley.com/doi/10.1111/bph.2010.159.issue-5/issuetoc
Keywords: signalosome, GPCR, constitutive activity, relaxin, concentration-biased, signalling, cAMP
The physiological relevance and consequence of cAMP generation is wide and varied, with this classical second messenger generating diverse, temporally and spatially specific actions. Increasingly, the enzymes responsible for cAMP generation themselves are implicated in multiple physiological roles, with knockout studies indicating an essential requirement of the adenylyl cyclases (ACs) in learning and memory, olfaction, cardiac contraction and alcohol addiction (reviewed in Sadana and Dessauer, 2009).
Compartmentalization of cAMP signalling generated by the formation of protein signalling complexes, or signalosomes, is a highly important emerging mechanism, whereby cAMP can affect numerous aspects of cell control in a specific and orchestrated manner (reviewed in Houslay, 2010; Pidoux and Taskén, 2010; Halls and Cooper, 2011). Despite the importance of this concept, the structural specificity of such signalosomes, and the relevance of the associated cAMP compartmentalization to downstream physiological responses, is poorly understood. Moreover, the evidence for the directed and specialized formation of these complexes in a cellular context is sparse.
The directed and specific compartmentalization of cAMP signalling, due to higher-order protein complexes, is a particularly important and relevant notion in the field of G-protein-coupled receptors (GPCRs; receptor nomenclature herein adheres to the recommendations published in the British Pharmacological Society's Guide to Receptors and Channels; Alexander et al., 2011). When considered within the environment of GPCR signalling, signalosome-mediated focussing of the spatial and temporal properties of an important second messenger such as cAMP significantly extends the established paradigms of GPCR compartmentalization, ligand-directed signalling and cell context-specific responses. Thus, the co-expression of a variety of cAMP-organizing entities in the same cellular compartment – including GPCRs, ACs and A-kinase anchoring proteins (AKAPs) – provides enormous scope for the specific and selective formation of ‘focused centres’ for cAMP signalling. These localized focal points not only facilitate the production of this classical second messenger but also allow the organization and scaffolding of effectors that are regulated by cAMP and the associated co-ordination of multiple regulatory elements. In this context, the relaxin family peptide receptor 1, RXFP1, is a GPCR that increasingly exemplifies the complexity and importance of compartmentalization and specificity in cAMP signalling.
The relaxin family peptide receptor, RXFP1, activates cAMP pathways to exert pleiotropic physiological responses
The RXFPs: a unique receptor family
The receptor for relaxin, the relaxin family peptide receptor 1 (RXFP1), is a GPCR of growing complexity that principally signals via multiple cAMP effectors. Relaxin is implicated in numerous physiological processes; initially discovered for its role during parturition in lower species (Hisaw, 1926), in humans, relaxin is now credited with an essential involvement in implantation and the maintenance of pregnancy during the first trimester (Stewart et al., 1990; Telgmann and Gellersen, 1998; Unemori et al., 1999; Bond et al., 2004; Hayes et al., 2004; Shirota et al., 2005). The effects of relaxin are far-reaching, with actions including vasodilation (Dschietzig et al., 2001; 2009a; Fisher et al., 2002; Novak et al., 2006; Teerlink et al., 2009; Teichman et al., 2010; Xu et al., 2010), direct effects on the heart (including increased inotropic and chronotropic responses; Kakouris et al., 1992; Dschietzig et al., 2009a; Teerlink et al., 2009), potent anti-fibrotic effects (Unemori et al., 1996; Garber et al., 2001; Mookerjee et al., 2006; Samuel et al., 2009), wound healing and increased angiogenesis (Unemori et al., 2000; Lewis et al., 2001), and a role for relaxin in enhanced invasiveness and tumour metastases during cancer (Binder et al., 2002; 2004; Hombach-Klonisch et al., 2006; Kamat et al., 2006; Thompson et al., 2006; Vinall et al., 2006; Feng et al., 2007).
There are four recently deorphanized GPCRs for the relaxin family peptides. The four RXFPs can be categorized into two quite distinct groups: RXFP1 (formerly LGR7) and RXFP2 (formerly LGR8; G-protein-coupled receptor affecting testis descent, GREAT; GPR106) are leucine-rich repeat-containing GPCRs (LGRs), that respond to relaxin and insulin/like peptide 3 (INSL3), respectively; whereas RXFP3 (formerly GPCR135; somatostatin- and angiontensin-like peptide receptor, SALPR) and RXFP4 (formerly GPCR142; GPR100) are small-peptide like GPCRs (with similarity to the somatostatin and angiotensin II receptors), that respond to the neuropeptide relaxin-3 and INSL5 respectively (Hsu et al., 2002; Kumagai et al., 2002; Liu et al., 2003; 2005). RXFP1 and RXFP2 are categorized as family C LGRs and resemble the glycoprotein hormone receptors – family A LGRs that include both the leuteinizing hormone, LH, and thyroid-stimulating hormone, TSH, receptors (Hsu et al., 2000; 2002). The family C LGRs are distinguished from other related receptors due to their unique extracellular domain, which encompasses a low-density lipoprotein class A (LDLa) module at the extreme N-terminus, followed by 10 leucine-rich repeats, leading into the transmembrane and C-terminal regions. The LGR family can be evolutionarily traced back to nematodes and insects (Hsu et al., 2000), and as such is thought to represent one of the earliest forms of GPCR signalling (Bathgate et al., 2006). Despite their early divergence, the receptors are far from simple in terms of their activation and regulatory mechanisms, with evidence accumulating for multiple ligand binding sites (Sudo et al., 2003; Halls et al., 2005), receptor dimerization (Kern et al., 2008), negative co-operativity (Svendsen et al., 2008a,b) and a conspicuous lack of significant desensitization and internalization (Callander et al., 2009; Kern and Bryant-Greenwood, 2009) that distinguishes them from the more prototypical GPCRs.
Activation of the relaxin receptor, RXFP1, occurs in a complex fashion, involving binding of ligand to both high- and low-affinity binding sites (Sudo et al., 2003; Halls et al., 2005; Svendsen et al., 2008a,b) and an as yet undefined interaction of the LDLa domain to facilitate signalling (Scott et al., 2006; Hopkins et al., 2007; Kern et al., 2007). Relaxin activation of RXFP1 results in stimulation of multiple signal transduction pathways including cAMP (influenced by a variety of Gα isoforms, discussed below), extracellular signal-regulated kinases (ERK) (Zhang et al., 2002; Dschietzig et al., 2003; 2009b; Mookerjee et al., 2009), tyrosine kinases (Palejwala et al., 1998; Kuznetsova et al., 1999; Bartsch et al., 2001; Anand-Ivell et al., 2007; Heng et al., 2008) and nitric oxide signalling (reviewed in Nistri and Bani, 2003; Conrad and Novak, 2004), in addition to the activation of signalling pathways associated with connective tissue metabolism (including inhibition of transforming growth factor-β signalling and activation of matrix metalloproteinase production; Unemori and Amento, 1990; Unemori et al., 1996; Bennett et al., 2003; Masterson et al., 2004; Samuel et al., 2004; Heeg et al., 2005; Ho et al., 2007) and direct stimulation of the glucocorticoid receptor (Dschietzig et al., 2009b,c).
RXFP1 exerts physiological effects via cAMP
Despite evidence for relaxin-activation of multiple second messengers, the major signalling pathway activated by relaxin (and the most studied to date) is associated with the stimulation of cAMP accumulation. In studies that pre-dated receptor identification, relaxin was found to increase cAMP accumulation in a number of cells and tissues, including THP-1 cells (Parsell et al., 1996), MCF-7 cells (Bigazzi et al., 1992), uterine tissue from rodents (Sanborn et al., 1980; Osa et al., 1991), cultures of human endometrial cells (Chen et al., 1988; Fei et al., 1990) and rat anterior pituitary cells (Cronin et al., 1987). Furthermore, following the discovery of a receptor for relaxin, mutants of RXFP1 were generated based on the conserved behaviour of constitutively active LH and TSH receptors (Hsu et al., 2000); mutant LH and TSH receptors identified in patients with male-limited precocious puberty and non-immune hyperthyroidism, respectively, contain point mutations in transmembrane domain 6 that are associated with constitutive activity (Parma et al., 1993; 1997; Shenker et al., 1993; Laue et al., 1995; 1996; Kosugi et al., 1996; 1998). Within this region, a ‘Phe-Thr-Asp’ motif is completely conserved between the glycoprotein hormone receptors and RXFP1; subsequent mutation of the last residue in this motif engendered constitutive activity in RXFP1 (Asp637Tyr) that was associated with increased cAMP accumulation (Hsu et al., 2000). However, not all cells yield robust increases in cAMP in response to relaxin. Stimulation of rat ventricular fibroblasts or rat renal myofibroblasts causes only weak and transient cAMP production (Samuel et al., 2004; Mookerjee et al., 2009), and there is no increase in cAMP following peptide stimulation of human lower uterine segment fibroblasts (Palejwala et al., 1998; 2001).
Despite these subtle inconsistencies, the physiological relevance of a cAMP response to relaxin is well demonstrated. In human endometrial stromal cells, basal and relaxin-stimulated cAMP levels are enhanced by inhibition of phosphodiesterase (PDE) 4, and relaxin stimulation and PDE4 inhibition act synergistically to induce decidualization (Bartsch et al., 2004), an important process required to support implantation of the developing embryo. Such increases in cAMP in endometrial cell cultures in response to relaxin (Huang et al., 1987; Tabanelli et al., 1992) mimic the addition of a cell permeable cAMP analogue (Tang et al., 1993). Indeed, only relaxin and cAMP itself are able to stimulate the decidualization of endometrial stromal cells in vitro in the absence of progesterone (Callander et al., 2009). Increases in cAMP mediated by relaxin are also linked to the physiological effects of the peptide upon angiogenesis; treatment of a murine model with human relaxin increased the degree of angiogenesis at wound sites, which was associated with an increased expression of vascular endothelial growth factor (VEGF), an important pro-angiogenic protein (Unemori et al., 2000). Interestingly, in cultures of normal human endometrial cells (NHE cells), human relaxin also increased the expression of VEGF, and these effects of relaxin were prevented by AC inhibition, and mimicked by either the AC activator forskolin or a PDE inhibitor (Unemori et al., 1999). This suggests that relaxin-stimulated cAMP production mediates increased VEGF transcription and, consequently, angiogenesis. The positive inotropic effects of relaxin on the atrial myocardium (Kakouris et al., 1992; Ward et al., 1992) are also linked to activation of cAMP pathways; the increased inotropy induced by relaxin was completely abolished by a PKA inhibitor (Piedras-Rentería et al., 1997a,b; Dschietzig et al., 2011), or an inhibitor of the rapidly inactivating component of the transient K+ outward current (Ito, carried by the Kv4.3 channel; Piedras-Rentería et al., 1997a,b; Dschietzig et al., 2011), and partially inhibited by a phosphatidylinositol 3-kinase (PI3K; Dschietzig et al., 2011) or Gαi/o inhibitor (Kompa et al., 2002; Dschietzig et al., 2011). This suggests that the cAMP generated via the Gαi/o–PI3K pathway (see below) facilitates PKA-phosphorylation of Kv4.3, leading to increased Ca2+ influx and thus increased inotropy. To this end, relaxin is currently in clinical trials for its efficacy in acute heart failure. Clearly, cAMP signalling is a very important and central mechanism, whereby relaxin exerts multiple physiological outcomes.
Multiplicity in relaxin-stimulated cAMP signalling generates great physiological potential, controlled by differential G-protein coupling, compartmentalization of cellular responses and concentration-biased agonism
The molecular identity of the proteins involved in generating cAMP downstream of RXFP1 activation has been the focus of many recent studies. Although this research has revealed the complexity of the cAMP pathways activated by RXFP1, principally due to the promiscuous coupling of the receptor to different Gα isoforms (RXFP1 couples to Gαs, Gαi3 and GαoB, which together can both stimulate and inhibit AC activity via different mechanisms; generally, these G-proteins can also affect Ca2+ channel, K+ channel, phospholipase C and phospholipase A2 activity), it has also suggested great scope for the pleiotropic physiological effects mediated by relaxin.
Differential G-protein coupling is directed by the cellular context of RXFP1 expression
Upon receptor activation, RXFP1 couples to Gαs, which stimulates AC activity and results in increased cAMP production (Hsu et al., 2000; 2002; Halls et al., 2006). Recent studies suggest that the interaction between RXFP1 and Gαs occurs within the third intracellular loop. A peptide derived from this loop (residues 615–629; Figure 2) increased AC activity independently of RXFP1 stimulation, and functionally antagonized receptor activation (Shpakov et al., 2007). This observation is also consistent with the gain-of-function receptor mutants (described above) that constitutively increase cAMP following a point mutation in the adjacent transmembrane 6 (Hsu et al., 2000; Figure 2). In addition to Gαs activation, RXFP1 also couples to GαoB, which inhibits AC activity (Halls et al., 2006; 2009a; Mookerjee et al., 2009). Additional complexity in cAMP accumulation is engendered by the simultaneous coupling of RXFP1 to Gαi3, which activates a further surge of cAMP accumulation via a Gβγ-PI3K-protein kinase C (PKC) ζ pathway to specifically activate AC5 (Nguyen et al., 2003; Nguyen and Dessauer, 2005a,b; Halls et al., 2006; 2009a). Activation of this Gαi3 pathway is dependent upon the final 10 amino acids of the RXFP1 C-terminal tail (requiring Arg752; Figure 2) and localization of the receptor within lipid-rich membrane domains (Halls et al., 2009a).
Figure 2.
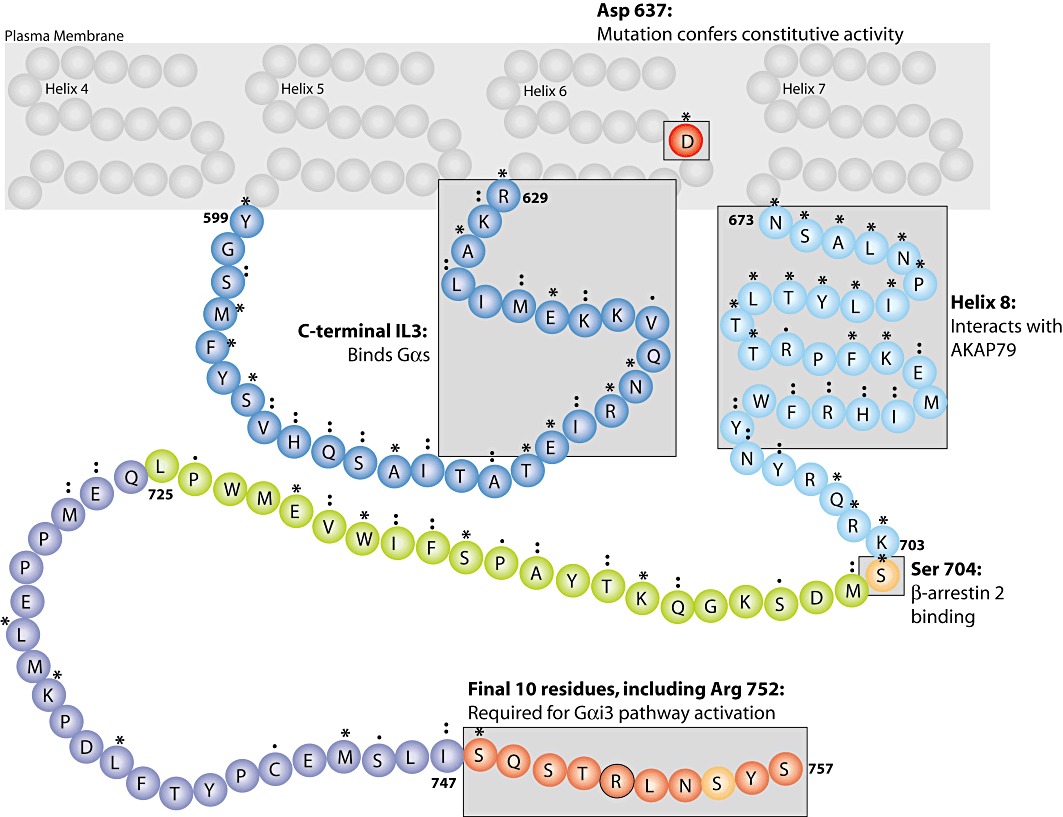
The importance of the third intracellular loop and C-terminus of RXFP1 in cAMP signalling. Many important regions for activation of cAMP signalling pathways within the intracellular loops and C-terminal tail of RXFP1 have now been identified. The third intracellular loop and transmembrane domain 6 are important for the activation of cAMP signalling pathways: the C-terminal portion of the third intracellular loop (residues 615–629) is suggested to couple to Gαs, and mutation of Asp637 induces a constitutive activation of cAMP signalling. The first section of the C-terminal tail regulates the formation of the RXFP1-signalosome: putative helix 8 (residues 673–703) is required for the interaction between RXFP1 and AKAP79 (which scaffolds AC2), and Ser704 is absolutely required for the interaction between the C-terminus and β-arrestin 2 (anchoring the regulatory sub-complex). The final 10 residues of the C-terminus (748–757), and specifically Arg752 are absolutely required for activation of the Gαi3–Gβγ–PI3K–PKCζ–AC5 cAMP signalling pathway. The RXFP1 C-terminal tail is not palmitoylated. Symbols above the amino acid residues indicate the relative conservation between RXFP1 and RXFP2: * identical residue, conserved residue and semi-conserved residue.
While RXFP1 simultaneously couples to all three pathways when expressed in HEK293 cells, endogenous expression of RXFP1 appears to allow more selective Gα isoform coupling in a manner dependent upon cell type. Endogenous expression of RXFP1 in primary cultures of rat cardiac fibroblasts and THP-1 cell lines allows receptor coupling to all three G-protein pathways linked to cAMP that were originally described in HEK293 cells: GαS, GαoB and Gαi3 (Halls et al., 2009b; Halls and Cooper, 2010). In distinct cellular backgrounds – when RXFP1 is endogenously expressed in primary cultures of rat renal myofibroblasts or Colo16 cell lines – the receptor couples only to the Gαs and GαoB pathways and is thus unable to activate the additional and sustained increase in cAMP mediated by Gαi3 signalling (Halls et al., 2009b; Mookerjee et al., 2009). Even greater functional limitation occurs in a T-47D cell line, where endogenous RXFP1 is only able to couple to Gαs to affect cAMP accumulation (Halls et al., 2009b).
Clearly, the cellular context of receptor expression dictates the degree of potential pleiotropy in G-protein coupling, thereby either greatly limiting or extending the functionality of a particular receptor system. This cell-directed variation in receptor functionality has far-reaching consequences in the associated activation of downstream cellular responses. Recently, this has been clearly demonstrated in human atrial myocardium from failing hearts (Dschietzig et al., 2011). In non-failing atrial myocardium, the positive inotropic effects of relaxin were partially inhibited by Gαi/o and PI3K inhibitors; however, in failing myocardium, the response was completely abolished (Dschietzig et al., 2011). This coincided with a significant (approximately 200%) increase in Gαi3 expression levels (Dschietzig et al., 2011) despite a slight decrease in RXFP1 expression (Kompa et al., 2002); in fact, the failing heart is classically characterized by increased expression of Gαi proteins and unchanged expression of Gαs (Eschenhagen et al., 1992; Böhm et al., 1994), which is deemed responsible for compromised β2-adrenoceptor–cAMP signalling (Rau et al., 2003). Thus, differential expression levels of Gαi3 in the same tissue manifested by disease pathogenesis can shift the principal mediators of the RXFP1–cAMP response, from Gαs to Gαi3-focussed cAMP pathways.
Compartmentalization of cellular responses by a single second messenger
The coupling of RXFP1 to combinations of Gαs, GαoB or Gαi3 dependent upon the cellular context allows scope for differential degrees of cAMP pathway activation and thus diverse cellular responses. Further scope for diversity in the cellular response to relaxin activation of RXFP1 derives from compartmentalization of the cAMP pool downstream of receptor coupling to G-proteins. Thus, Gαs and GαoB pathways affect cAMP-response element (CRE)-controlled gene transcription, whereas the Gαi3 pathway does not; in addition, only the Gαi3 pathway can affect nuclear factor of κB (NFκB)-mediated gene transcription (Halls et al., 2007). The physiological consequence of this selective and specific gene transcription initiated by the different G-protein pathways influencing cAMP accumulation is yet to be demonstrated. However, taken together within the context of differential G-protein–cAMP pathway activation dependent upon a particular cellular background, this observation provides further rationalization for the pleiotropy of relaxin's physiological actions.
Additionally, the opposing activity of Gαs and GαoB upon a single second messenger, and associated downstream transcriptional regulation, suggests that the levels of cAMP stimulated by relaxin–mediated activation of RXFP1 are tightly regulated and maintained within a defined range depending on the cellular background. Thus in HEK293 cells over-expressing RXFP1, or indeed in THP-1 cells, the Gαs response tends to overwhelm the inhibition exerted by GαoB (Halls et al., 2006; 2009a,b). Conversely, in HeLa cells or primary cultures of rat cardiac fibroblasts, the inhibition of AC activity exerted by pertussis toxin-sensitive Gαi/o proteins overwhelms the Gαs-mediated AC stimulation, and concentration-dependent increases in cAMP in response to relaxin are only revealed following pertussis toxin treatment (Halls and Cooper, 2010). Thus, even though cells may not increase cAMP in response to relaxin, a small degree of AC stimulation could be functionally masked due to the overwhelming effect of Gαi/o inhibition. This raises the interesting possibility that this signalling pathway could be highly susceptible to hijacking by other GPCRs or viral systems (Nijmeijer et al., 2010; Tschische et al., 2010), reversing the relaxin-mediated cellular effects to a more Gαs-dominant signalling system and thus affecting physiological endpoints.
Concentration-biased agonism: a role for circulating relaxin?
The coupling of RXFP1 to the Gαs, GαoB and Gαi3 cAMP signalling pathways, and indeed the physiological effects attributed to this ligand-receptor pair, occurs in response to nanomolar concentrations of relaxin, with EC50 values typically reported in the 0.1–0.5 nM range (Sudo et al., 2003; Halls et al., 2005; 2006; Summers et al., 2010). Consequently, relaxin is thought to mediate its physiological actions in an autocrine or paracrine manner, in order to achieve the concentrations required for receptor activation. In addition to this localized relaxin production and release, there is also evidence for the secretion of relaxin into the circulation; however, in this capacity, the ligand is only present at concentrations much lower than those currently established to generate effective cellular responses (Sherwood, 2004). In fact, the measurement of relaxin in the circulation is hampered by the sensitivity limits of elisa assays, and thus, the peptide may actually be present in much smaller amounts than presently thought (Sherwood, 2004). Although there is as yet no specific physiological role attributed to circulating relaxin, vanishingly low concentrations of the peptide can generate a unique cellular response; this occurs via an RXFP1–signalosome that specifically responds to sub-picomolar concentrations of relaxin (Halls and Cooper, 2010). As such, this unique role for circulating compared to locally synthesized relaxin demonstrates a new paradigm of concentration-biased agonism in this receptor system (Figure 1).
Figure 1.
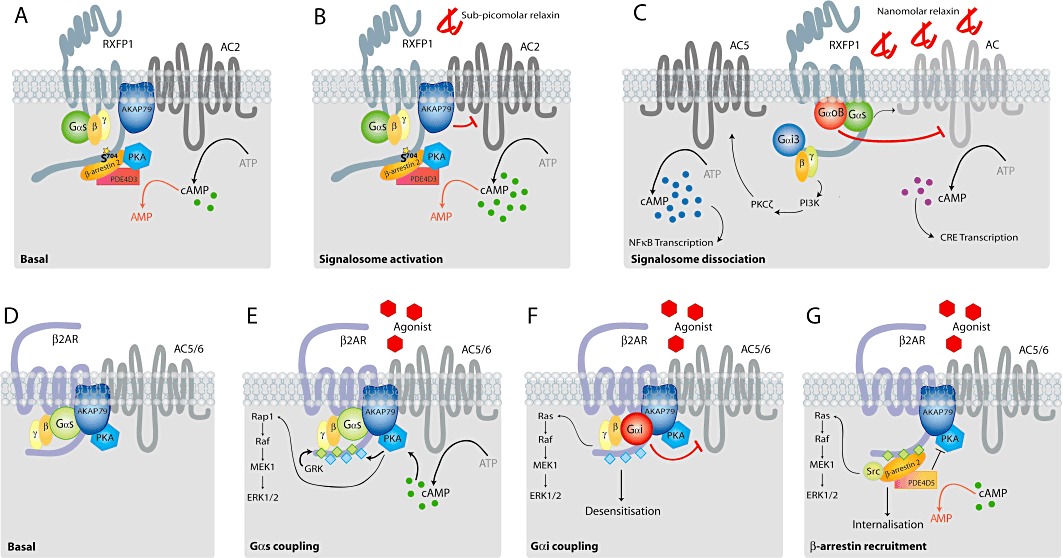
Concentration-biased signalling at the relaxin receptor, RXFP1, compared with prototypical β2-adrenoceptor signalling. The relaxin receptor RXFP1 demonstrates differential activation of intracellular signalling pathways, leading to increased cAMP accumulation in response to increasing concentrations of ligand; this is in contrast to the prototypical activation, desensitization and internalization paradigm demonstrated by the β2-adrenoceptor. A. Under basal conditions, expression of RXFP1 induces the formation of an active signalosome. AKAP79 interacts with helix 8 of the RXFP1 C-terminal tail and thereby scaffolds AC2 to the vicinity of the receptor; this allows efficient activation of AC2 by both Gαs and Gβγ-subunits. The cAMP generated by the stimulation of AC2 is tightly controlled by the activity of PDE4D3. This phosphodiesterase is activated by PKA (in a negative feedback loop) and additionally interacts with β-arrestin 2, which binds to Ser704 of the RXFP1 C-terminus, thereby anchoring the regulatory proteins to the signalosome. B. Sub-picomolar concentrations of relaxin (down to attomolar levels) further activate the pre-assembled, receptor-driven signalosome. The cAMP generated following signalosome activation is maintained within a tightly defined range by the activity of PDE4D3, and this is probably complemented by direct AKAP79-mediated inhibition of AC2 activity. C. Increasing concentrations of relaxin appear to induce dissociation of the RXFP1-signalosome (Halls and Cooper, 2010). At nanomolar concentrations, relaxin stimulation of RXFP1 activates the classical cAMP signalling pathways. RXFP1 can couple to Gαs to activate AC, and GαoB, which inhibits AC activity. The cAMP generated by the combined influence of these two G-protein pathways, ultimately affects CRE-controlled gene transcription. RXFP1 can additionally couple to Gαi3, which activates a Gβγ-PI3K-PKCζ pathway, resulting in increased AC5 activity. The additional and sustained increases in cAMP generated by this pathway will only affect NFκB-mediated gene transcription. D. Under basal conditions, the β2-adrenoceptor is also associated with AKAP79, which scaffolds AC5/6 and PKA to the vicinity of the receptor. There is no evidence for cAMP turnover within this complex. E. Following receptor stimulation, the liberation of Gαs activates AC5/6 to increase cAMP. This results in PKA activation, which phosphorylates the receptor, and can also activate ERK1/2 signalling. The activated receptor can be phosphorylated by GRKs. F. PKA phosphorylation of the receptor C-terminus, results in an uncoupling or signal switching of the β2-adrenoceptor from Gαs to Gαi, leading to receptor desensitization. The Gβγ subunits liberated from Gαi can also activate ERK1/2 signalling. G. GRK phosphorylation of the receptor C-terminus leads to β-arrestin recruitment and receptor internalization. Recruitment of β-arrestin also allows the scaffolding of Src and PDE4D5 proteins: Src can activate ERK1/2 signalling; whereas PDE4D5 hydrolyses cAMP and inhibits the activity of PKA, preventing signal switching to Gαi and facilitating desensitization.
GPCR–signalosomes: high-resolution signalling systems
An RXFP1-signalosome facilitates attomolar ligand responses, constitutive activity and concentration-biased agonism
Upon expression of RXFP1, the receptor induces the formation of a constitutively active and tightly regulated signalosome, which constitutes a highly sensitive mechanism whereby attomolar concentrations of relaxin can induce cAMP accumulation. RXFP1 is scaffolded to AC2 via AKAP79, allowing efficient activation of the AC by Gαs and Gβγ subunits. The cAMP produced is tightly regulated by the activity of protein kinase A (PKA)-activated PDE4D3, scaffolded to the receptor C-terminus (specifically requiring Ser704) by β-arrestin 2 (Halls and Cooper, 2010). The stimulatory (AKAP79 and AC2) and regulatory (β-arrestin 2, PKA and PDE4D3) components of the signalosome appear to be both spatially and functionally distinct (Figure 2): knockdown of AKAP79 does not affect the association between the regulatory components and the receptor, whereas knockdown of β-arrestin 2 does not prevent the interactions between RXFP1 and the stimulatory components. Furthermore, the protein constituents of the complex exhibit isoform selectivity, determined using targeted protein knockdown or over-expression of dominant negative mutants (Halls and Cooper, 2010): thus, of the PKA-activated PDE4D isoforms expressed in HEK293 cells (reviewed in Houslay, 2010), the complex is specific for PDE4D3; assembly of the regulatory components is dependent upon a constitutive association between the receptor and β-arrestin 2, but not β-arrestin 1 (reviewed in DeFea, 2011); and the cAMP activity of the RXFP1-signalosome is dependent upon the constitutive association between helix 8 of the receptor and AKAP79, but not gravin (AKAP250) or AKAP149 (reviewed in Baillie et al., 2005; Dessauer, 2009; Skroblin et al., 2010). Importantly, and uniquely, this signalling mechanism is absolutely distinct from the cAMP signalling pathways activated by higher concentrations of relaxin; indeed, the complex appears to dissociate following activation of RXFP1 with nanomolar concentrations of peptide. Supra-picomolar concentrations of relaxin instead increase cAMP as previously described, via Gαs stimulation of AC, negatively modulated by the inhibitory activity of GαoB, with a sustained, further rise in cAMP generated via a Gαi3–Gβγ–PI3K–PKCζ–AC5 pathway (Hsu et al., 2002; Nguyen et al., 2003; Nguyen and Dessauer, 2005a,b; Halls et al., 2006; 2009a).
Relaxin is therefore a concentration-biased agonist at RXFP1; activation of RXFP1 by relaxin proceeds by a biphasic concentration–response mechanism (Figure 1) with increases in cAMP detected following stimulation with as little as 10 aM of peptide. This degree of sensitivity has been demonstrated in only a few other physiological systems, including the suppression of pro-inflammatory cytokine production by interleukin-15 (Alleva et al., 1997), proliferation of a helper T-cell line by interleukin-1 (Orencole and Dinarello, 1989), the effects of neuropeptides and neurosteroids in nociception (Sánchez-Blázquez and Garzón, 1995; Ueda et al., 2001) and the long-term effects of transforming growth factor-β on basal FSH levels (Ying et al., 1986). Activation of a GPCR however does not typically occur in response to such small concentrations of ligand. Thus, the specific assembly of a GPCR–signalosome facilitates a substantially increased sensitivity of the receptor system to ligand, and these protein complexes thereby constitute high-resolution signalling systems.
A particularly interesting feature of the RXFP1–signalosome is the paradigm of concentration-biased signalling exhibited by the pre-assembled proteins. Concentration-biased signalling can be considered analogous to the well-established concept of ligand-biased signalling, whereby a ligand preferentially activates a specific signalling pathway; similarly, in this case a particular concentration of ligand activates a specific signalling pathway. Thus, at concentrations below the nanomolar level, relaxin appears only to stimulate a small increase in cAMP via the signalosome, whereas at supra-nanomolar concentrations, relaxin preferentially activates the classical relaxin signalling pathways affecting cAMP accumulation, defined by Gαs, GαoB and Gαi3. Importantly, there is no effect of inhibitors of classical pathway-specific proteins (including Gαi/o, PI3K and PKC) on cAMP generated in response to sub-picomolar concentrations of relaxin. The reciprocal also holds – there is no effect of inhibition of signalosome-specific proteins (including AC2, AKAP79 and β-arrestin 2) upon classical relaxin cAMP signalling. Furthermore, higher concentrations of relaxin appear to dissociate the signalosome, thereby allowing activation of the classical signalling pathways. Thus, the signalosome and classical relaxin signalling pathways appear to be quite distinct in composition. It is also interesting to consider the potential contrast of these concentration-specific signalling pathways in terms of lipid raft dependence. It is established that AC2 is preferentially excluded from lipid-rich domains (reviewed in Willoughby and Cooper, 2007), whereas activation of the Gαi3 pathway following supra-nanomolar relaxin stimulation of RXFP1 depends upon lipid-rich domains in HEK293 cells (Halls et al., 2009a).
The physiological application of this highly sensitive, concentration-dependent signalosome is not yet apparent. However, it is intriguing to consider this complex within the context of the role of relaxin during embryo implantation. In a number of tissues, relaxin exerts its effects over an extended period of time, exemplified by endometrial decidualization (the differentiation of endometrial stromal cells), which is crucial for embryo implantation and the maintenance of pregnancy. Both circulating and locally produced relaxin have been implicated in this process (Einspanier et al., 1999; Unemori et al., 1999; Palejwala et al., 2002; Hayes et al., 2004), which requires the continued elevation of cAMP (reviewed in Telgmann and Gellersen, 1998; Gellersen and Brosens, 2003). Thus, the sensitivity of RXFP1 to sub-picomolar concentrations of relaxin may suggest a mechanism that extends a physiological, perhaps homeostatic, role for the concentrations of peptide present within the circulation and thus may be linked to some of the long-term physiological effects of this hormone. Future research, perhaps utilizing relaxin knockout models or single cell models of physiologically relevant targets (i.e. atrial cardiomyocytes, endometrial cells, fibroblasts or neurons), will be required to fully elucidate the downstream consequences and physiological significance of this ultra-sensitive cAMP signalling pathway.
Physiological relevance of attomolar-agonism: an essential link to AC2?
Increased cAMP production in response to sub-picomolar concentrations of relaxin was found to be specific for, and dependent upon, the expression of AC2. AC2 belongs to the group II ACs (including AC2, AC4 and AC7) that are defined by their conditional stimulation by Gβγ-subunits (dependent upon co-activation by Gαs) and activation by PKC phosphorylation (reviewed in Halls and Cooper, 2011). In addition to AC2, HEK293 cells also endogenously express AC7 from this same sub-group of ACs. However, over-expression of AC7 abolished the sub-picomolar relaxin response, which directly opposed the enhancement of sub-picomolar cAMP signalling observed following AC2 over-expression. Thus, the RXFP1-controlled signalosome is specific for the expression of AC2 and consequently may not exist in all cell types.
AC2 is principally expressed in brain, lung, skeletal muscle, heart and uterus (myometrium) (reviewed in Defer et al., 2000; Willoughby and Cooper, 2007; Sadana and Dessauer, 2009), as well as the spinal cord (Ehnert et al., 2004). Within the spinal chord, ACs are thought to have a role in the pain response (Sadana and Dessauer, 2009), a system that is sensitive to attomolar concentrations of stimuli (Sadana and Dessauer, 2009). There is also evidence for the expression and function of AC2 in the olfactory system, another system that is designed to be highly sensitive to physiological stimuli. Within the olfactory system, AC2 expression predominates in the vomeronasal system, which is responsible for pheromone detection, rather than the odorant-detecting main olfactory epithelium, where the expression of AC3 dominates (Sadana and Dessauer, 2009). Both the pain response within the spinal chord and the detection of pheromones within the vomeronasal system are dependent upon the cAMP produced by AC2 and respond to very low concentrations of stimuli. Based on these observations it is tantalizing to speculate that a signalosome specific for AC2 could be present and be required for these sensitive physiological responses. In terms of the RXFP1–signalosome, there is potential for the expression of AC2 and RXFP1 to overlap in the brain, lung, heart and uterus, and this may suggest a high likelihood for a physiological role of this specific signalosome in these tissues.
The importance of AKAPs in cAMP–signalosomes: scaffolds and negative regulators of signalling
The high-sensitivity activation of AC2 within the RXFP1-signalosome is dependent upon the scaffolding of the AC to RXFP1 by AKAP79. The AKAPs constitute a large and growing family of highly divergent proteins, with the exception of a conserved PKA interaction motif (reviewed in Wong and Scott, 2004). Due to the very limited homology between AKAPs, a general AC–AKAP interaction motif has not been identified. In fact, it appears that different AKAPs use disparate mechanisms to interact with the same AC, and further, that different ACs bind to disparate regions of the same AKAP (reviewed in Dessauer, 2009). Nonetheless, AKAPs have an emerging importance in directing compartmentalization and control of the sub-cellular localization and molecular specificity for targets of cAMP signalling pathways. This is achieved by their action as scaffolds, not only for ACs but also by tethering cAMP effectors, downstream targets and regulatory proteins.
AKAP79 is a scaffold for calcineurin, PKC, GPCRs (including the β1- and β2-adrenoceptors, AMPA ionotropic glutamate receptors and the relaxin receptor RXFP1) and a variety of channels [including the voltage-activated Ca2+ channel Cav1.4 (L-type channel), voltage-activated K+ channel family Kv7 (M-type/KCNQ channels) and the transient receptor potential cation channel TRPV1] (reviewed in Dessauer, 2009; Halls and Cooper, 2010). More recent studies have also demonstrated the ability of AKAP79 to anchor a number of AC isoforms, including AC2, AC3, AC5, AC6, AC8 and AC9 (Bauman et al., 2006; Efendiev et al., 2010; Willoughby et al., 2010). By this multivalent activity, AKAP79 can therefore potentially direct the differential regulation of distinct protein complexes merely by its scaffolding function.
However, the influence of AKAP79 (on ACs at least) extends further than its ability to act as a protein scaffold. In fact, AKAP79 has been demonstrated to interact with and affect the activity of most AC isoforms. The association of AKAP79 with both AC5 and AC6 results in the facilitation of PKA-mediated AC phosphorylation and a subsequent inhibition of AC activity (Bauman et al., 2006). Similarly, the anchoring of AC8 by AKAP79 in both over-expression and endogenous neuronal systems limits the sensitivity of the AC to activation by Ca2+ (Willoughby et al., 2010). In a similar manner, the activity of AC2 is also inhibited by the presence of AKAP79 (Efendiev et al., 2010). However, AKAP79 merely acts as a neutral scaffold for AC3 and AC9 (Efendiev et al., 2010).
The role of AKAP79 in scaffolding AC2 to the RXFP1–signalosome appears to be of a dual nature. While AKAP79 may have a small degree of inhibitory influence on the activity of AC2, its principal role in this case appears to be the scaffolding of RXFP1 and AC2 within the complex. Thus, removal of AKAP79 from the system results in a loss of sub-picomolar cAMP signalling, rather than relieving any substantial negative influence upon AC activity.
A new GPCR regulatory paradigm demonstrated by signalosome formation: lack of desensitization and internalization, a constitutive association with β-arrestins and dual coupling to G-proteins
The regulatory sub-complex of the RXFP1–signalosome is dependent upon the association of β-arrestin 2 with Ser704 of the receptor C-terminal tail (Figure 2), which exclusively occurs following the expression of RXFP1 and is independent of ligand stimulation (Halls and Cooper, 2010). This contrasts the accepted paradigm for GPCR/β-arrestin interactions, which typically occur following receptor activation and phosphorylation (Figure 1). Indeed, although β-arrestin binding was traditionally thought to only occur following GRK or second messenger (i.e. PKA or PKC) phosphorylation of an activated receptor, it is now apparent that associations between GPCRs and β-arrestins can occur in the absence of both receptor activation and phosphorylation (reviewed in DeFea, 2011). Furthermore, the regulation of RXFP1 activity following stimulation with supra-nanomolar concentrations of relaxin does not appear to follow the prototypical desensitization and internalization paradigm described by studies of the β2-adrenoceptor (Callander et al., 2009; Kern and Bryant-Greenwood, 2009). This classical GPCR paradigm predicts that ligand stimulation of the receptor facilitates activation of G-proteins, phosphorylation of the receptor C-terminus by G-protein receptor kinases (GRKs), recruitment of β-arrestins (which uncouple the receptor from its G-protein partners) and receptor internalization, which corresponds with the associated activation of β-arrestin-mediated signalling pathways (Figure 1; reviewed in Kelly et al., 2008; Tobin et al., 2008; Rajagopal et al., 2010).
Well-established observations made prior to the deorphanization of RXFP1 suggested that the receptor was not desensitized; activation of the relaxin receptor in numerous target tissues was associated with prolonged physiological effects that persisted for longer than 6 h with constant washing (Summers et al., 1995; Tan et al., 1998). This observation is still consistently replicated in cell population and single cell signalling assays (albeit over smaller time scales; Halls ML, unpubl. obs.). Furthermore, recent evidence specifically looking for the prototypical GPCR regulatory features, suggested an absence of significant receptor internalization following ligand binding (Callander et al., 2009). Finally, the basal interaction between RXFP1 and β-arrestin 2, when considered in conjunction with the constitutive activity of the signalosome, suggests dual coupling of both Gαs and β-arrestin 2 to the intracellular regions of RXFP1. Again, this conflicts with the general paradigm of GPCR signalling, which suggests mutually exclusive coupling of a GPCR to either G-protein- or β-arrestin-mediated signalling pathways.
Interestingly, this alternate paradigm of constitutive β-arrestin association, dual G-protein coupling and an absence of appreciable receptor desensitization and internalization has also been demonstrated by another GPCR. The dopamine D4 receptor does not exhibit significant desensitization or internalization following stimulation with dopamine. Instead, this receptor constitutively co-immunoprecipitates with β-arrestin 2, and this association does not decrease following receptor stimulation (Rondou et al., 2010; Spooren et al., 2010), which classically activates Gαi/o proteins. Further examples of dual G-protein coupling are evident in other GPCR systems, which do exhibit typical internalization characteristics. The calcium-sensing (CaS) receptor is constitutively associated with β-arrestin 1, and this association does not change following agonist stimulation (Bouschet et al., 2007), despite the receptor coupling to Gαq/11, Gα12/13 and Gαi/o proteins (Brown et al., 2010). Furthermore, two mutants of the V2 vasopressin receptor (responsible for nephrogenis syndrome of inappropriate antidiuresis, NSIAD) exhibit constitutive cAMP accumulation, and BRET studies show a constitutive association between the receptors and β-arrestin 1 (Tenenbaum et al., 2009).
Taken together, these observations of the behaviour of RXFP1, the D4, CaS and V2 receptors, which conflict with the classical GPCR regulatory paradigm, may suggest a situation whereby constitutively active receptors that are basally associated with β-arrestins are also able to tolerate and coordinate dual coupling to G-proteins. Alternatively, the phosphorylation state of the target receptor could induce differential conformational states of β-arrestins, which subsequently dictates their function (Shenoy et al., 2006). A mutant of the β2-adrenoceptor, which lacks G-protein coupling but retains β-arrestin binding, exhibited weak receptor phosphorylation, moderate β-arrestin recruitment, but robust ERK1/2 phosphorylation in contrast to the wild-type receptor (Shenoy et al., 2006). The authors suggest that the β-arrestin 2 recruited to a GRK2-phosphorylated receptor may not be optimally suited for engaging efficient ERK1/2 activation (Shenoy et al., 2006). This raises the possibility of distinct β-arrestin 2 conformations, dictated by the receptor to which they are bound, that may also permit dual coupling of the receptor to G-proteins.
Interestingly, the RXFP1, D4 dopamine and CaS receptors all behave unexpectedly in terms of their constitutive associations with β-arrestins, when examined in imaging studies. In each case, confocal studies using GFP-tagged β-arrestins showed only cytosolic expression of the tagged protein, which did not change following receptor expression (which was localized to the plasma membrane) or ligand stimulation (Bouschet et al., 2007; Callander et al., 2009; Rondou et al., 2010; Spooren et al., 2010). This may suggest that a relatively small population of endogenously expressed β-arrestin is responsible for constitutive GPCR-interactions, or perhaps that prior recruitment of endogenous β-arrestins to the receptors subsequently prevents an exchange with the GFP-tagged variant following expression. Alternatively, the addition of the GFP-tag to the β-arrestin itself may interfere with the constitutive association between β-arrestins and these more unusual GPCRs. One might also add the caveat that in instances of over-expression, it may be difficult to see subtle recruitment of a cytosolic GFP-tagged protein to the plasma membrane unless expression levels of this protein are kept very low. Nonetheless, these receptors clearly display relatively unique interactions with β-arrestins.
The role of β-arrestins as independent and influential signalling scaffolds
The concept of β-arrestins acting as protein scaffolds in their own right is an emerging area that extends the role of these proteins, initially defined by their involvement in the prototypical desensitization and internalization of classical GPCRs (reviewed in DeWire et al., 2007; Ma and Pei, 2007; DeFea, 2008; 2011). Indeed, β-arrestins are often considered multifunctional adaptor proteins, with evidence accumulating for associations with numerous signalling mediators including those with roles in signal transduction (i.e. protein kinases, phosphatases, trafficking proteins, small G-proteins), metabolic enzymes, proteins implicated in cellular organization (i.e. cytoskeletal proteins, motor proteins), chaperone and stress response proteins, ion channels and nucleic acid binding proteins (i.e. transcription factor, RNA processing, DNA binding and ribosomal proteins) (Xiao et al., 2007).
In the context of the RXFP1–signalosome, it appears that β-arrestin 2 may be purely acting as a scaffolding protein, as there is still no evidence for β-arrestin 2-mediated activation of downstream signalling pathways in response to relaxin. Interestingly, this is in contrast to the role of β-arrestin 2 in the β2-adrenergic receptor complex; in this case, β-arrestin 2 recruitment to the GRK-phosphorylated C-terminus allows the associated recruitment of PDE4D5, which reverses the AKAP79/PKA-mediated switching of the receptor from Gαs-cAMP-PKA to Gαi/o-ERK (Houslay and Baillie, 2005) and facilitates subsequent β-arrestin 2 stimulation of ERK pathways. Thus, the principal function of β-arrestin may be controlled by the receptor to which it is bound. In this context, the type of proteins scaffolded by β-arrestin also appear to be influenced by the receptor in question; thus, the RXFP1/AKAP79/β-arrestin 2 complex has a preference for PDE4D3 (Halls and Cooper, 2010) compared with the β2-adrenoceptor/AKAP79/β-arrestin 2 complex that is specific for the PDE4D5 isoform (Bolger et al., 2003; Lynch et al., 2005; Willoughby et al., 2007). It is interesting to speculate that this specificity in recruitment of a particular isoform of PDE4D may be dependent upon the conformation of β-arrestin 2 induced by the interaction with a particular receptor C-terminus. Indeed, previous studies have shown that β-arrestin preferentially interacts with PDE4D5 over other PDE4 isoforms, even in cell types where PDE4D5 is not the predominant species (Houslay and Baillie, 2005), due to an additional interaction site for β-arrestins within the N-terminal region, separate from the site within the catalytic unit (Bolger et al., 2003). As such, the preference in the RXFP1-signalosome for PDE4D3 over PDE4D5 may be induced by the conformation of β-arrestin 2 adopted following binding to the RXFP1 C-terminus.
The concept of differential conformations of β-arrestins defined by their binding partners is not new; there is supportive evidence to suggest that β-arrestins may adopt different conformations with specific preferences for G-protein occupied versus unoccupied receptors, and other studies have demonstrated changes in β-arrestin binding partners following receptor activation (DeWire et al., 2007). These specific conformational (and thus functional) states of β-arrestins are also demonstrated in studies examining the effect of mutation of potential phosphorylation sites within the C-terminal tail of various GPCRs; removal of phosphorylation sites either ablates β-arrestin recruitment and associated ERK activation (i.e. β2-adrenoceptor), has no effect on the ability of the receptor to recruit β-arrestins and activate ERK phosphorylation (i.e. angiotensin II AT1A receptor) or can result in the receptor retaining the ability to recruit β-arrestins but losing any associated activation of ERK signalling pathways (i.e. orexin OX1 receptor) (DeWire et al., 2007).
Thus, in the context of GPCR-signalosomes, β-arrestins do function as truly flexible adaptor proteins, whereby the relevant conformation of the β-arrestin is initially defined by receptor binding, and subsequently dictates the additional scaffolding of specific proteins to the signalosome.
Physiological implications of signalling complexity: the RXFP1–signalosome
Many, if not all, GPCRs can intrinsically exhibit a degree of constitutive, or ligand-independent, activity (reviewed in Smit et al., 2007), and this has direct implications for the targeting of these receptors as therapeutics. The degree of constitutive activity exhibited by a particular receptor can be influenced by a specific ligand, or by point mutations, thus manipulating the balance between active and inactive receptor states. Correspondingly, variations in the constitutive activity of a receptor dependent upon the expression of the GPCR within different cell types, and associated variations in downstream signalling constituents, add an additional area of complexity; thus, a truncated CB1 cannabinoid receptor that exhibits enhanced constitutive activity results in an increase in inverse agonist efficacy (SR141716A) but a decrease in agonist efficacy (WIN 55,212–2) (Nie and Lewis, 2001). The relative constitutive activity of a receptor will also affect the outcome of the interaction between the GPCR and a particular ligand. Furthermore, variation in the constitutive activity of a GPCR raises the concept of protean agonism (reviewed in Kenakin, 2001), whereby ligands can exhibit positive agonism in a system with low constitutive activity, but inverse agonism in a system with a high degree of constitutive activity. Indeed, many important therapeutic agents, initially considered competitive antagonists, are now recognized to be inverse agonists with negative intrinsic activities, including the α1-adrenoceptor target prazosin and the dopamine D2 receptor ligand haloperidol (Smit et al., 2007).
Many wild-type receptors demonstrate constitutive activity in the absence of any disturbance in equilibrium caused by either ligand or mutation, and some such as the histamine H3 receptor actually require the presence of inverse agonists in order to maintain homeostasis (Morisset et al., 2000; Wieland et al., 2001). Similarly, many of the constitutively active wild-type GPCRs are receptors for neurotransmitters, which suggests a requirement of constitutive activity for the maintenance of neuronal tone (Seifert and Wenzel-Seifert, 2002). Thus, it is interesting to speculate such a homeostatic or neurotransmitter-like relevance for the RXFP1-signalosome, particularly in its physiological roles in decidualization and neuronal function. Furthermore, the identification of RXFP1 as a receptor with intrinsic constitutive activity, in the absence of any influence of a ligand or mutational manipulations, has interesting consequences for the interpretation of its known physiological roles and for the potential development of therapeutics targeting this system. Additional complexity arises when natural (due to expression of RXFP1 within different cell types) or induced (hijaacking of this signalling system by other receptors) variations in the expression of signalling constituents are considered, and this may perhaps reveal a cellular mechanism for the pleitropy of relaxin's physiological effects.
However, signalling complexity is not only derived from the constitutive activity exhibited by the RXFP1–signalosome but can also be attributed to the cAMP signalling bias that is directed by increasing concentrations of ligand. On a cellular level, the potential physiological consequences of specific cAMP pools generated in response to different concentrations of agonist, and by temporally distinct pathways, are vast. This not only implies a fine degree of spatial and temporal control over the cellular signal but also greatly increases the range of cellular responses that may be activated by a single ligand/receptor pair. This, in addition to other amplifying factors including ligand-directed signalling, and differences in protein composition between varying cell types, makes transparent the pleiotropic physiological effects of this interesting hormone. The physiological relevance of a highly sensitive GPCR–signalosome, and whether this protein complex functions purely in a homeostatic realm, remains to be determined. Nevertheless, the directed assembly of a finely tuned GPCR–signalosome that facilitates concentration-biased agonism would dramatically increase the physiological potential of any GPCR system. Future research is essential to discover how widespread the phenotype displayed by the RXFP1–signalosome is.
Acknowledgments
MLH is a National Health and Medical Research Council of Australia Overseas Biomedical Fellow (519581). The author thanks Prof Dermot MF Cooper for valuable critical revision of the manuscript.
Glossary
- AC
adenylyl cyclase
- AKAP
A-kinase anchoring protein
- CRE
cAMP response element
- GRK
G-protein-coupled receptor kinase
- INSL
insulin/relaxin-like peptide
- LDLa
low-density lipoprotein class A
- LGR
leucine-rich repeat-containing GPCR
- LH
leuteinizing hormone
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- RXFP
relaxin family peptide receptor
- TSH
thyroid-stimulating hormone
Conflict of interest
The author states no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva DG, Kaser SB, Monroy MA, Fenton MJ, Beller DI. IL-15 functions as a potent autocrine regulator of macrophage proinflammatory cytokine production: evidence for differential receptor subunit utilization associated with stimulation or inhibition. J Immunol. 1997;159:2941–2951. [PubMed] [Google Scholar]
- Anand-Ivell R, Heng K, Bartsch O, Ivell R. Relaxin signalling in THP-1 cells uses a novel phosphotyrosine-dependent pathway. Mol Cell Endocrinol. 2007;272:1–13. doi: 10.1016/j.mce.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod. 2001;7:799–809. doi: 10.1093/molehr/7.9.799. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:324–334. doi: 10.1210/jc.2003-030498. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International union of pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006;58:7–31. doi: 10.1124/pr.58.1.9. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RG, Kharbanda KK, Tuma DJ. Inhibition of markers of hepatic stellate cell activation by the hormone relaxin. Biochem Pharmacol. 2003;66:867–874. doi: 10.1016/s0006-2952(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Bigazzi M, Brandi ML, Bani G, Sacchi TB. Relaxin influences the growth of MCF-7 breast cancer cells. Mitogenic and antimitogenic action depends on peptide concentration. Cancer. 1992;70:639–643. doi: 10.1002/1097-0142(19920801)70:3<639::aid-cncr2820700316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Binder C, Hagemann T, Husen B, Schultz M, Einspanier A. Relaxin enhances in vitro invasiveness of breast cancer cell lines by upregulation of matrix metalloproteinases. Mol Hum Reprod. 2002;8:789–796. doi: 10.1093/molehr/8.9.789. [DOI] [PubMed] [Google Scholar]
- Binder C, Simon A, Binder L, Hagemann T, Schulz M, Emons G, et al. Elevated concentrations of serum relaxin are associated with metastatic disease in breast cancer patients. Breast Cancer Res Treat. 2004;87:157–166. doi: 10.1023/B:BREA.0000041622.30169.16. [DOI] [PubMed] [Google Scholar]
- Böhm M, Eschenhagen T, Gierschik P, Larisch K, Lensche H, Mende U, et al. Radioimmunochemical quantification of Giα in right and left ventricles from patients with ischaemic and dilated cardiomyopathy and predominant left ventricular failure. J Mol Cell Cardiol. 1994;26:133–149. doi: 10.1006/jmcc.1994.1017. [DOI] [PubMed] [Google Scholar]
- Bolger GB, McCahill A, Huston E, Cheung YF, McSorley T, Baillie GS, et al. The unique amino-terminal region of the PDE4D5 cAMP phosphodiesterase isoform confers preferential interaction with β-arrestins. J Biol Chem. 2003;278:49230–49238. doi: 10.1074/jbc.M303772200. [DOI] [PubMed] [Google Scholar]
- Bond CP, Parry LJ, Samuel CS, Gehring HM, Lederman FL, Rogers PAW, et al. Increased expression of the relaxin receptor (LGR7) in the human endometrium during the secretory phase of the menstrual cycle. J Clin Endocrinol Metab. 2004;89:3477–3485. doi: 10.1210/jc.2003-030798. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Kanamarlapudi V, Mundell S, Henley JM. The calcium-sensing receptor changes cell shape via a β-arrestin-1 ARNO ARF6 ELMO protein network. J Cell Sci. 2007;120:2489–2497. doi: 10.1242/jcs.03469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Brauner-Osborne H, Bikle D, Conigrave A, Shoback D. 2010. Calcium-sensing receptors. Last modified on 2010-06-29. Accessed on 2011-01-10. IUPHAR Database (IUPHAR-DB), http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=12.
- Callander GE, Thomas WG, Bathgate RA. Prolonged RXFP1 and RXFP2 signaling can be explained by poor internalization and a lack of β-arrestin recruitment. Am J Physiol Cell Physiol. 2009;296:C1058–C1066. doi: 10.1152/ajpcell.00581.2008. [DOI] [PubMed] [Google Scholar]
- Chen GA, Huang JR, Tseng L. The effect of relaxin on cyclic adenosine 3′,5′-monophosphate concentrations in human endometrial glandular epithelial cells. Biol Reprod. 1988;39:519–525. doi: 10.1095/biolreprod39.3.519. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Novak J. Emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol. 2004;287:R250–R261. doi: 10.1152/ajpregu.00672.2003. [DOI] [PubMed] [Google Scholar]
- Cronin MJ, Malaska T, Bakhit C. Human relaxin increases cyclic AMP levels in cultured anterior pituitary cells. Biochem Biophys Res Commun. 1987;148:1246–1251. doi: 10.1016/s0006-291x(87)80266-8. [DOI] [PubMed] [Google Scholar]
- DeFea KA. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol. 2008;153(Suppl. 1):S298–S309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA. Beta-arrestins as regulators of signal termination and transduction: hoe do they determine what to scaffold? Cell Signal. 2011;23:621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- Dessauer CW. Adenylyl cyclase – a-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76:935–941. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, et al. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15:2187–2195. doi: 10.1096/fj.01-0070com. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist. Attenutation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-κB. Circ Res. 2003;92:32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, et al. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail. 2009a;15:182–190. doi: 10.1016/j.cardfail.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Baumann G, Stangl K. RXFP1-inactive relaxin activates human glucocorticoid receptor: further investigations into the relaxin-GR pathway. Regul Pept. 2009b;154:77–84. doi: 10.1016/j.regpep.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Wessler S, Baumann G, Stangl K. Autoregulation of human relaxin-2 gene expression critically involves relaxin and glucocorticoid receptor binding to glucocorticoid response half-sites in the relaxin-2 promoter. Regul Pept. 2009c;155:163–173. doi: 10.1016/j.regpep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Alexiou K, Kinkel H-T, Baumann G, Matschke K, Stangl K. The positive inotropic effect of relaxin-2 in human atrial myocardium is preserved in end-stage heart failure: role of Gi-phosphoinositide-3 kinase signalling. J Card Fail. 2011;17:158–166. doi: 10.1016/j.cardfail.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Samelson BK, Nguyen BT, Phatarpekar PV, Baameur F, Scott JD, et al. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem. 2010;285:14450–14458. doi: 10.1074/jbc.M110.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehnert C, Tegeder I, Pierre S, Birod K, Nguyen HV, Schmidtko A, et al. Protein associated with Myc (PAM) is involved in spinal nociceptive processing. J Neurochem. 2004;88:948–957. doi: 10.1046/j.1471-4159.2003.02229.x. [DOI] [PubMed] [Google Scholar]
- Einspanier A, Nubbemeyer R, Schlote S, Schumacher M, Ivell R, Fuhrmann K, et al. Relaxin in the marmoset monkey: secretion pattern in the ovarian cycle and early pregnancy. Biol Reprod. 1999;61:512–520. doi: 10.1095/biolreprod61.2.512. [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Mende U, Nose M, Schmitz W, Scholz H, Haverich A, et al. Increased messenger RNA level of the inhibitory G protein α subunit Gαi2 in human end-stage heart failure. Circ Res. 1992;70:688–696. doi: 10.1161/01.res.70.4.688. [DOI] [PubMed] [Google Scholar]
- Fei DT, Gross MC, Lofgren JL, Mora-Worms M, Chen AB. Cyclic AMP response to recombinant human relaxin by cultured human endometrial cells – a specific and high throughput in vitro bioassay. Biochem Biophys Res Commun. 1990;170:214–222. doi: 10.1016/0006-291x(90)91262-q. [DOI] [PubMed] [Google Scholar]
- Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, et al. Relaxin promotes prostate cancer progression. Clin Cancer Res. 2007;13:1695–1702. doi: 10.1158/1078-0432.CCR-06-2492. [DOI] [PubMed] [Google Scholar]
- Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, et al. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart? Circulation. 2002;106:292–295. doi: 10.1161/01.cir.0000025630.05387.45. [DOI] [PubMed] [Google Scholar]
- Garber SL, Mirochnik Y, Brecklin CS, Unemori EN, Singh AK, Slobodskoy L, et al. Relaxin decreases renal interstitial fibrosis and slows progression of renal disease. Kidney Int. 2001;59:876–882. doi: 10.1046/j.1523-1755.2001.059003876.x. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Halls ML, Cooper DM. Sub-picomolar relaxin signalling by a pre-assembled RXFP1, AKAP79, AC2, β-arrestin 2, PDE4D3 complex. EMBO J. 2010;29:2772–2787. doi: 10.1038/emboj.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3:a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls ML, Bond CP, Sudo S, Kumagai J, Ferraro T, Layfield S, et al. Multiple binding sites revealed by interaction of relaxin family peptides with native and chimeric relaxin family peptide receptors 1 and 2 (LGR7 and LGR8) J Pharmacol Exp Ther. 2005;313:677–687. doi: 10.1124/jpet.104.080655. [DOI] [PubMed] [Google Scholar]
- Halls ML, Bathgate RAD, Summers RJ. Relaxin family peptide receptors, RXFP1 and RXFP2, modulate cAMP signalling by distinct mechanisms. Mol Pharmacol. 2006;70:214–226. doi: 10.1124/mol.105.021691. [DOI] [PubMed] [Google Scholar]
- Halls ML, Bathgate RAD, Summers RJ. Comparison of signalling pathways activated by the relaxin family peptide receptors, RXFP1 and RXFP2, using reporter genes. J Pharmacol Exp Ther. 2007;320:281–290. doi: 10.1124/jpet.106.113225. [DOI] [PubMed] [Google Scholar]
- Halls ML, van der Westhuizen ET, Wade JD, Evans BA, Bathgate RA, Summers RJ. Relaxin family peptide receptor (RXFP1) coupling to Gαi3 involves the C-terminal Arg752 and localization within membrane raft microdomains. Mol Pharmacol. 2009a;75:415–428. doi: 10.1124/mol.108.051227. [DOI] [PubMed] [Google Scholar]
- Halls ML, Hewitson TD, Moore XL, Du XJ, Bathgate RA, Summers RJ. Relaxin activates multiple cAMP signaling pathway profiles in different target cells. Ann N Y Acad Sci. 2009b;1160:108–111. doi: 10.1111/j.1749-6632.2008.03814.x. [DOI] [PubMed] [Google Scholar]
- Hayes ES, Curnow EC, Trounson AO, Danielson LA, Unemori EN. Implantation and pregnancy following in vitro fertilization and the effect of recombinant human relaxin administration in Macaca fascicularis. Biol Reprod. 2004;71:1591–1597. doi: 10.1095/biolreprod.104.030585. [DOI] [PubMed] [Google Scholar]
- Heeg MHJ, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA, et al. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int. 2005;68:96–109. doi: 10.1111/j.1523-1755.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- Heng K, Ivell R, Wagaarachchi P, Anand-Ivell R. Relaxin signalling in primary cultures of human myometrial cells. Mol Hum Reprod. 2008;14:603–611. doi: 10.1093/molehr/gan051. [DOI] [PubMed] [Google Scholar]
- Hisaw FL. Experimental relaxation of the pubic ligament of the guinea-pig. Proc Soc Exp Biol Med. 1926;23:661–663. [Google Scholar]
- Ho TY, Yan W, Bagnell CA. Relaxin-induced matrix metalloproteinase-9 expression is associated with activation of the NF-κB pathway in human THP-1 cells. J Leukoc Biol. 2007;81:1303–1310. doi: 10.1189/jlb.0906556. [DOI] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Bialek J, Trojanowicz B, Weber E, Holzhausen H-J, Silvertown JD, et al. Relaxin enhances the oncogenic potential of human thyroid carcinoma cells. Am J Pathol. 2006;169:617–632. doi: 10.2353/ajpath.2006.050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins EJ, Layfield S, Ferraro T, Bathgate RAD, Gooley PR. The NMR solution structure of the relaxin (RXFP1) receptor lipoprotein receptor class A module and identification of key residues in the N-terminal region of the module that mediate receptor activation. J Biol Chem. 2007;282:4172–4184. doi: 10.1074/jbc.M609526200. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS. β-arrestin-recruited phosphodiesterase-4 desensitizes the AKAP79/PKA-mediated switching of β2-adrenoceptor signalling to activation of ERK. Biochem Soc Trans. 2005;33:1333–1336. doi: 10.1042/BST0331333. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, et al. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Huang JR, Tseng L, Bischof P, Janne OA. Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology. 1987;121:2011–2017. doi: 10.1210/endo-121-6-2011. [DOI] [PubMed] [Google Scholar]
- Kakouris H, Eddie LW, Summers RJ. Cardiac effects of relaxin in rats. Lancet. 1992;339:1076–1078. doi: 10.1016/0140-6736(92)90665-p. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Feng S, Agoulnik IU, Kheradmand F, Bogatcheva NV, Coffey D, et al. The role of relaxin in endometrial cancer. Cancer Biol Ther. 2006;5:71–77. doi: 10.4161/cbt.5.1.2289. [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008;153(Suppl. 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- Kern A, Bryant-Greenwood GD. Characterization of relaxin receptor (RXFP1) desensitization and internalization in primary human decidual cells and RXFP1-transfected HEK293 cells. Endocrinology. 2009;150:2419–2428. doi: 10.1210/en.2008-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Agoulnik AI, Bryant-Greenwood GD. The low-density lipoprotein class a module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology. 2007;148:1181–1194. doi: 10.1210/en.2006-1086. [DOI] [PubMed] [Google Scholar]
- Kern A, Hubbard D, Amano A, Bryant-Greenwood GD. Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing g protein-coupled receptor 7) splice variants from human fetal membranes. Endocrinology. 2008;149:1277–1294. doi: 10.1210/en.2007-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompa AR, Samuel CS, Summers RJ. Inotropic responses to human gene 2 (B29) relaxin in a rat model of myocardial infarction (MI): effect of pertussis toxin. Br J Pharmacol. 2002;137:710–718. doi: 10.1038/sj.bjp.0704922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Mori T, Shenker A. The role of Asp578 in maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. J Biol Chem. 1996;271:31813–31817. doi: 10.1074/jbc.271.50.31813. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Mori T, Shenker A. An anionic residue at position 564 is important for maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. Mol Pharmacol. 1998;53:894–901. [PubMed] [Google Scholar]
- Kumagai J, Hsu SY, Matsumi H, Roh J-S, Fu P, Wade JD, et al. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277:31283–31286. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- Kuznetsova L, Plesneva S, Derjabina N, Omeljaniuk E, Pertseva M. On the mechanism of relaxin action: the involvement of adenylyl cyclase signalling system. Regul Pept. 1999;80:33–39. doi: 10.1016/s0167-0115(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Laue L, Chan WY, Hsueh AJ, Kudo M, Hsu SY, Wu SM, et al. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci USA. 1995;92:1906–1910. doi: 10.1073/pnas.92.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue L, Wu SM, Kudo M, Hsueh AJ, Cutler GB, Jr, Jelly DH, et al. Heterogeneity of activating mutations of the human luteinizing hormone receptor in male-limited precocious puberty. Biochem Mol Med. 1996;58:192–198. doi: 10.1006/bmme.1996.0048. [DOI] [PubMed] [Google Scholar]
- Lewis M, Deshpande U, Gizman L, Grove BH, Huang X, Erikson ME. Systemic relaxin administration stimulates angiogenic cytokine expression and vessel formation in a rat myocardial infarct model. In: Tregear GW, Ivell R, Bathgate RA, Wade JD, et al., editors. Relaxin 2000, Proceedings of the Third Internation Conference on Relaxin and Related Peptides. Broome, Australia: Kluwer Academic Publishers; 2001. pp. 159–168. [Google Scholar]
- Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, et al. Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem. 2003;278:50754–50764. doi: 10.1074/jbc.M308995200. [DOI] [PubMed] [Google Scholar]
- Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, Wu J, et al. INSL5 is a high affinity specific agonist for GPCR142 (GPR100) J Biol Chem. 2005;280:292–300. doi: 10.1074/jbc.M409916200. [DOI] [PubMed] [Google Scholar]
- Lynch MJ, Baillie GS, Mohamed A, Li X, Maisonneuve C, Klussmann E, et al. RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with β-arrestin to control the protein kinase A/AKAP79-mediated switching of the β2-adrenergic receptor to activation of ERK in HEK293B2 cells. J Biol Chem. 2005;280:33178–33189. doi: 10.1074/jbc.M414316200. [DOI] [PubMed] [Google Scholar]
- Ma L, Pei G. β-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, et al. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant. 2004;19:544–552. doi: 10.1093/ndt/gfg598. [DOI] [PubMed] [Google Scholar]
- Mookerjee I, Solly NR, Royce SG, Tregear GW, Samuel CS, Tang MLK. Endogenous relaxin regulates collagen deposition in an animal model of allergic airway disease. Endocrinology. 2006;147:754–761. doi: 10.1210/en.2005-1006. [DOI] [PubMed] [Google Scholar]
- Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, et al. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J. 2009;23:1219–1229. doi: 10.1096/fj.08-120857. [DOI] [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Dessauer CW. Relaxin stimulates protein kinase C ζ translocation: requirement for cyclic adenosine 3′,5′-monophosphate production. Mol Endocrinol. 2005a;19:1012–1023. doi: 10.1210/me.2004-0279. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Dessauer CW. Relaxin stimulates cAMP production in MCF-7 cells upon overexpression of type V adenylyl cyclase. Ann N Y Acad Sci. 2005b;1041:296–299. doi: 10.1196/annals.1282.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BT, Yang L, Sanborn BM, Dessauer CW. Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3′,5′-monophosphate by relaxin. Mol Endocrinol. 2003;17:1075–1084. doi: 10.1210/me.2002-0284. [DOI] [PubMed] [Google Scholar]
- Nie J, Lewis DL. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J Neurosci. 2001;21:8758–8764. doi: 10.1523/JNEUROSCI.21-22-08758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijmeijer S, Leurs R, Smit MJ, Vischer HF. The Epstein-Barr virus-encoded G protein-coupled receptor BILF1 hetero-oligomerizes with human CXCR4, scavenges Gαi proteins, and constitutively impairs CXCR4 functioning. J Biol Chem. 2010;285:29632–29641. doi: 10.1074/jbc.M110.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri S, Bani D. Relaxin receptors and nitric oxide synthases: search for the missing link. Reprod Biol Endocrinol. 2003;1:5–6. doi: 10.1186/1477-7827-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, et al. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006;20:2352–2362. doi: 10.1096/fj.06-6263com. [DOI] [PubMed] [Google Scholar]
- Orencole SF, Dinarello CA. Characterization of a subclone (D10S) of the D10.G4.1 helper T-cell line which proliferates to attomolar concentrations of interleukin-1 in the absence of mitogens. Cytokine. 1989;1:14–22. doi: 10.1016/1043-4666(89)91044-2. [DOI] [PubMed] [Google Scholar]
- Osa T, Inoue H, Okabe K. Effects of porcine relaxin on contraction, membrane response and cyclic AMP content in rat myometrium in comparison with the effects of isoprenaline and forskolin. Br J Pharmacol. 1991;104:950–960. doi: 10.1111/j.1476-5381.1991.tb12532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palejwala S, Stein D, Wojtczuk A, Weiss G, Goldsmith LT. Demonstration of a relaxin receptor and relaxin-stimulated tyrosine phosphorylation in human lower uterine segment fibroblasts. Endocrinology. 1998;139:1208–1212. doi: 10.1210/endo.139.3.5772. [DOI] [PubMed] [Google Scholar]
- Palejwala S, Stein DE, Weiss G, Monia BP, Tortoriello D, Goldsmith LT. Relaxin positively regulates metrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signalling pathway. Endocrinology. 2001;142:3405–3413. doi: 10.1210/endo.142.8.8295. [DOI] [PubMed] [Google Scholar]
- Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod. 2002;66:1743–1748. doi: 10.1095/biolreprod66.6.1743. [DOI] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Hermans J, Rocmans P, Van Vliet G, et al. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thyroid adenomas. J Clin Endocrinol Metab. 1997;82:2695–2701. doi: 10.1210/jcem.82.8.4144. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Mak JY, Amento EP, Unemori EN. Relaxin binds to and elicits a response from cells of the human monocytic cell line, THP-1. J Biol Chem. 1996;271:27936–27941. doi: 10.1074/jbc.271.44.27936. [DOI] [PubMed] [Google Scholar]
- Pidoux G, Taskén K. Specificity and spatial dynamics of protein kinase A signalling organised by A-kinase-anchoring proteins. J Mol Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- Piedras-Rentería ES, Sherwood OD, Best PM. Effects of relaxin on rat atrial myocytes. I. Inhibition of Ito via PKA-dependent phosphorylation. Am J Physiol. 1997a;272:H1791–H1797. doi: 10.1152/ajpheart.1997.272.4.H1791. [DOI] [PubMed] [Google Scholar]
- Piedras-Rentería ES, Sherwood OD, Best PM. Effects of relaxin on rat atrial myocytes. II. Increased calcium influx derived from action potential prolongation. Am J Physiol. 1997b;272:H1798–H1803. doi: 10.1152/ajpheart.1997.272.4.H1798. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau T, Nose M, Remmers U, Weil J, Wiessmüller A, Davia K, et al. Overexpression of wild-type Gαi2 suppresses β-adrenergic signalling in cardiac myocytes. FASEB J. 2003;17:523–525. doi: 10.1096/fj.02-0660fje. [DOI] [PubMed] [Google Scholar]
- Rondou P, Skieterska K, Packeu A, Lintermans B, Vanhoenacker P, Vauquelin G, et al. KLHL12-mediated ubiquitination of the dopamine D4 receptor does not target the receptor for degradation. Cell Signal. 2010;22:900–913. doi: 10.1016/j.cellsig.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, et al. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology. 2004;145:4125–4133. doi: 10.1210/en.2004-0209. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Royce SG, Chen B, Cao H, Gossen JA, Tregear GW, et al. Relaxin family peptide receptor-1 protects against airway fibrosis during homeostasis but not against fibrosis associated with chronic allergic airways disease. Endocrinology. 2009;150:1495–1502. doi: 10.1210/en.2008-1062. [DOI] [PubMed] [Google Scholar]
- Sanborn BM, Kuo HS, Weisbrodt NW, Sherwood OD. The interaction of relaxin with the rat uterus. I. Effect on cyclic nucleotide levels and spontaneous contractile activity. Endocrinology. 1980;106:1210–1215. doi: 10.1210/endo-106-4-1210. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Garzón J. αN-acetyl-β-endorphin-(1-31) disrupts the diminishing effect of mastoparan on opioid- and clonidine-evoked supraspinal antinociception in mice. J Pharmacol Exp Ther. 1995;273:787–792. [PubMed] [Google Scholar]
- Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJW, Tregear GW, et al. Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem. 2006;281:34942–34954. doi: 10.1074/jbc.M602728200. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, Minegishi T, Cutler GB., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- Shirota K, Tateishi K, Koji T, Hishikawa Y, Hachisuga T, Kuroki M, et al. Early human preantral follicles have relaxin and relaxin receptor (LGR7), and relaxin promotes their development. J Clin Endocrinol Metab. 2005;90:516–521. doi: 10.1210/jc.2004-0130. [DOI] [PubMed] [Google Scholar]
- Shpakov AO, Gur'yanov IA, Kuznetsova LA, Plesneva SA, Shpakova EA, Vlasov GP, et al. Studies of the molecular mechanisms of action of relaxin on the adenylyl cyclase signaling system using synthetic peptides derived from the LGR7 relaxin receptor. Neurosci Behav Physiol. 2007;37:705–714. doi: 10.1007/s11055-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Skroblin P, Grossmann S, Schäfer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase A anchoring. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, et al. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- Spooren A, Rondou P, Debowska K, Lintermans B, Vermeulen L, Samyn B, et al. Resistance of the dopamine D4 receptor to agonist-induced internalization and degradation. Cell Signal. 2010;22:600–609. doi: 10.1016/j.cellsig.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Stewart DR, Celniker AC, Taylor CAJ, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990;70:1771–1773. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem. 2003;278:7855–7862. doi: 10.1074/jbc.M212457200. [DOI] [PubMed] [Google Scholar]
- Summers RJ, Tan YY, Kakouris H, Eddie LW. Cardiac actions of relaxin. In: Maclennan AH, Tregear GW, Bryant-Greenwood GD, editors. Progress in Relaxin Research. Singapore: Global Publications Services; 1995. pp. 487–498. [Google Scholar]
- Summers RJ, Bathgate RAD, Ivell R, Sanborn BM, Sherwood OD. 2010. Relaxin family peptide receptors. Last modified on 2010-07-01. Accessed on 2011-01-02. IUPHAR database (IUPHAR-DB), http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=60.
- Svendsen AM, Vrecl M, Ellis TM, Heding A, Kristensen JB, Wade JD, et al. Cooperative binding of insulin-like peptide 3 to a dimeric relaxin family peptide receptor 2. Endocrinology. 2008a;149:1113–1120. doi: 10.1210/en.2007-0412. [DOI] [PubMed] [Google Scholar]
- Svendsen AM, Zalesko A, Konig J, Vrecl M, Heding A, Kristensen JB, et al. Negative cooperativity in H2 relaxin binding to a dimeric relaxin family peptide receptor 1. Mol Cell Endocrinol. 2008b;296:10–17. doi: 10.1016/j.mce.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol. 1992;42:337–344. doi: 10.1016/0960-0760(92)90137-8. [DOI] [PubMed] [Google Scholar]
- Tan YY, Wade JD, Tregear GW, Summers RJ. Comparison of relaxin receptors in rat isolated atria and uterus by use of synthetic and native relaxin analogues. Br J Pharmacol. 1998;123:762–770. doi: 10.1038/sj.bjp.0701659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Guller S, Gurpide E. Cyclic adenosine 3′,5′-monophosphate induces prolactin expression in stromal cells isolated from human proliferative endometrium. Endocrinology. 1993;133:2197–2203. doi: 10.1210/endo.133.5.8404671. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep. 2010;7:75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgmann R, Gellersen B. Marker genes of decidualisation: activation of the decidual prolactin gene. Hum Reprod Update. 1998;4:472–479. doi: 10.1093/humupd/4.5.472. [DOI] [PubMed] [Google Scholar]
- Tenenbaum J, Ayoub MA, Perkovska S, Adra-Delenne AL, Mendre C, Ranchin B, et al. The constitutively active V2 receptor mutants conferring NSIAD are weakly sensitive to agonist and antagonist regulation. PLoS ONE. 2009;4:e8383. doi: 10.1371/journal.pone.0008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson VC, Morris TG, Cochrane DR, Cavanagh J, Wafa LA, Hamilton T, et al. Relaxin becomes upregulated during prostate cancer progression to androgen independence and is negatively regulated by androgens. Prostate. 2006;66:1698–1709. doi: 10.1002/pros.20423. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Butcher AJ, Kong KC. Location, location, location . . . site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschische P, Moser E, Thompson D, Vischer HF, Parzmair GP, Pommer V, et al. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11:660–674. doi: 10.1111/j.1600-0854.2010.1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Yoshida A, Mizuno K, Yamamoto H, Maruo J, et al. Metabotropic neurosteroid/sigma-receptor involved in stimulation of nociceptor endings of mice. J Pharmacol Exp Ther. 2001;298:703–710. [PubMed] [Google Scholar]
- Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265:10681–10685. [PubMed] [Google Scholar]
- Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98:2739–2745. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14:800–806. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Lewis M, Constant J, Arnold G, Grove BH, Normand J, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000;8:361–370. doi: 10.1111/j.1524-475x.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- Vinall RL, Tepper CG, Shi X-B, Xue LA, Gandour-Edwards R, de Vere White RW. The R273H p53 mutation can facilitate the androgen-independent growth of LNCaP by a mechanism that involves H2 relaxin and its cognate receptor LGR7. Oncogene. 2006;25:2082–2093. doi: 10.1038/sj.onc.1209246. [DOI] [PubMed] [Google Scholar]
- Ward DG, Thomas GR, Cronin MJ. Relaxin increases rat heart rate by a direct action on the cardiac atrium. Biochem Biophys Res Commun. 1992;186:999–1005. doi: 10.1016/0006-291x(92)90845-c. [DOI] [PubMed] [Google Scholar]
- Wieland K, Bongers G, Yamamoto Y, Hashimoto T, Yamatodani A, Menge WM, et al. Constitutive activity of histamine H3 receptors stably expressed in SK-N-MC cells: display of agonism and inverse agonism by H3 antagonists. J Pharmacol Exp Ther. 2001;299:908–914. [PubMed] [Google Scholar]
- Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Baillie GS, Lynch MJ, Ciruela A, Houslay MD, Cooper DMF. Dynamic regulation, desensitisation, and cross-talk in discrete subcellular microdomains during β2-adrenoceptor and prostanoid receptor cAMP signalling. J Biol Chem. 2007;282:34235–34249. doi: 10.1074/jbc.M706765200. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Masada N, Wachten S, Pagano M, Halls ML, Everett KL, et al. AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem. 2010;285:20328–20342. doi: 10.1074/jbc.M110.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, et al. Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chakravorty A, Bathgate RA, Dart AM, Du XJ. Relaxin therapy reverses large artery remodeling and improves arterial compliance in senescent spontaneously hypertensive rats. Hypertension. 2010;55:1260–1266. doi: 10.1161/HYPERTENSIONAHA.109.149369. [DOI] [PubMed] [Google Scholar]
- Ying SY, Becker A, Baird A, Ling N, Ueno N, Esch F, et al. Type β transforming growth factor (TGF-β) is a potent stimulator of the basal secretion of follicle stimulating hormone (FSH) in a pituitary monolayer system. Biochem Biophys Res Commun. 1986;135:950–956. doi: 10.1016/0006-291x(86)91020-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu S-H, Erikson M, Lewis M, Unemori E. Relaxin activates the MAP kinase pathway in human endometrial stromal cells. J Cell Biochem. 2002;85:536–544. doi: 10.1002/jcb.10150. [DOI] [PubMed] [Google Scholar]


