Figure 1.
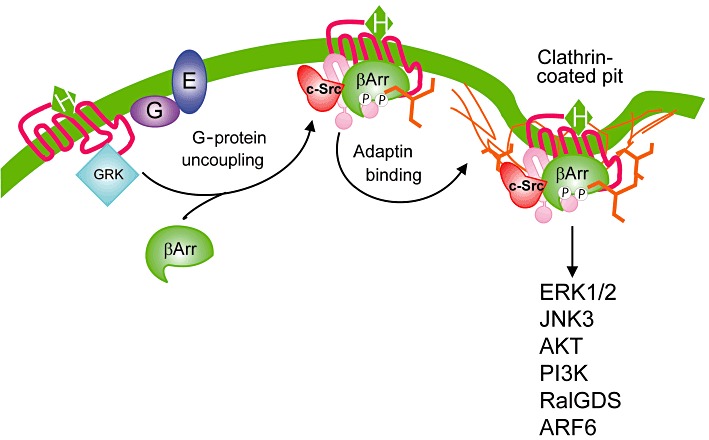
β-Arrestin-dependent endocytosis and signalling of GPCRs. Agonist activation promotes the GRK2-mediated phosphorylation that promotes the translocation and binding of β-arrestins, which serves to uncouple receptors from heterotrimeric G-proteins. β-Arrestins function as adaptor proteins that interact with both clathrin and β2-adaptin promoting the clathrin coated vesicle-mediated endocytosis of many GPCRs. c-Src is recruited to GPCRs as a consequence of its interaction with β-arrestin and couples the receptor to the MAPK pathway. β-Arrestin interactions with a variety of proteins allows for the coupling of GPCRs to a variety of different signal transduction pathways whose activation may be independent of heterotrimeric G-proteins. β-Arr, β-arrestin; P, phosphate.
