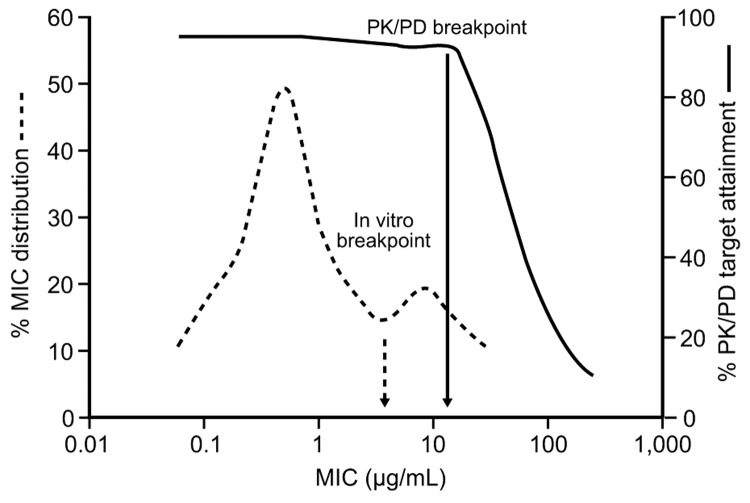
Figure 3.
Relationship between MIC and attainment of the pharmacokinetic/pharmacodynamic (PK/PD) target for effect. Accumulating evidence supports the use of separate PK/PD breakpoints for clinical decision making, distinct from in vitro breakpoints used for epidemiologic surveillance. A breakpoint derived from PK/PD data represents the highest MIC for which the unbound plasma concentrations of the drug (after standard doses) are sufficient to achieve the target PK/PD exposure.