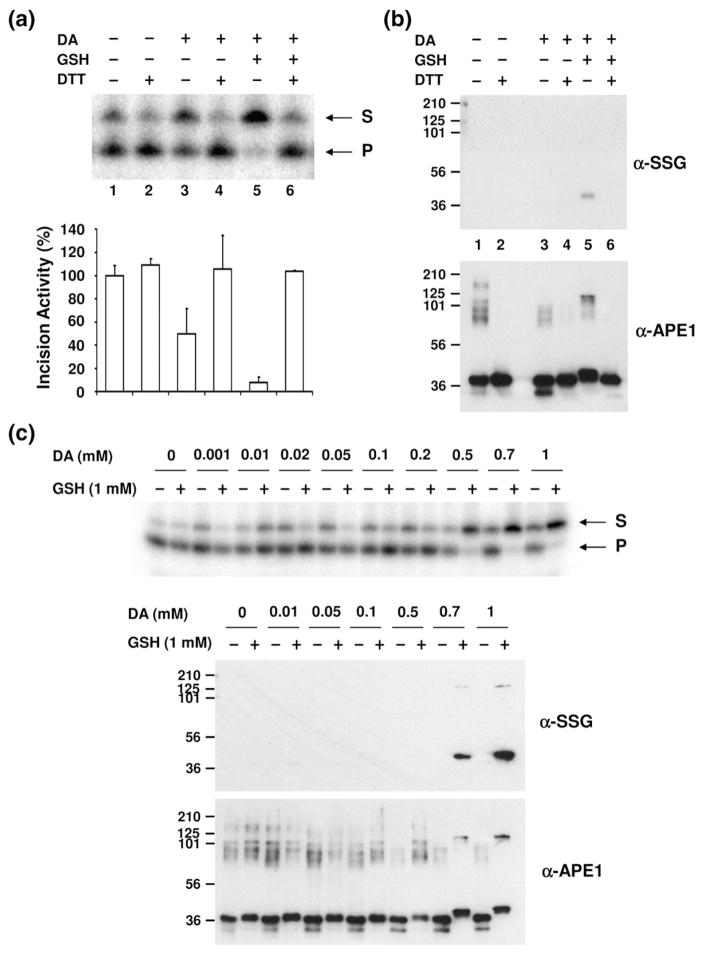
Fig. 2.
Effect of S-glutathionylation of APE1 on its AP endonuclease activity. (a) AP endonuclease activity of modified APE1 protein. Recombinant APE1 (0.2 μg) was incubated with 1 mM diamide (DA)/2 mM GSH, followed by 5 mM DTT treatment as indicated. AP-site-containing DNA substrate was incubated with unmodified or modified APE1 protein under standard reaction conditions (see Materials and Methods), and the reaction mixture was analyzed on a denaturing urea-polyacrylamide gel. S indicates uncleaved oligonucleotide substrates, and P indicates cleaved incision products. The graph represents the relative incision activity (compared to APE1 without DA, GSH, or DTT treatment) with means±SD (n=3). (b) The same samples assayed for AP endonuclease activity (a) were subjected to Western blot analysis. Equal amounts of proteins were separated by nonreducing SDS-PAGE and probed with antibodies specific to SSG or APE1. Lane designations 1–6 correspond to those in (a). Positions of molecular mass protein standards are designated in kilodaltons. The data are representative of at least three independent experiments. (c) Inhibition of AP endonuclease activity was observed by the addition of various concentrations of diamide (DA) together with 1 mM GSH (upper panel). The same samples were subjected to Western blot analysis with anti-SSG and anti-APE1 antibodies after separation by nonreducing SDS-PAGE (lower panel).