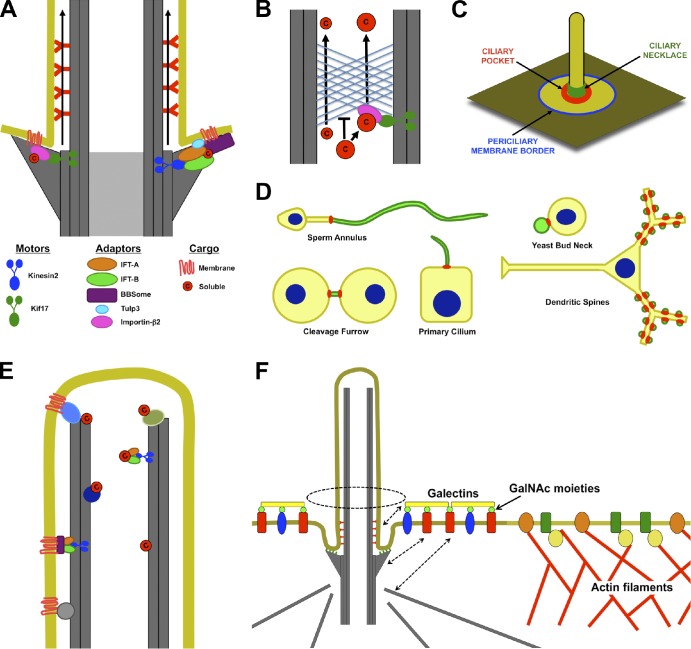
Figure 4.
Access into and maintenance of distinct ciliary compartments. (A) Both soluble and transmembrane proteins may need to associate directly or indirectly with plus end–directed microtubule motors (Kinesin-2 or Kif17) to enter the cilium. The entrance may be between adjacent transition fibers. (B) Like the nuclear pore, the ciliary base can exclude proteins on the basis of their size. Thus, small proteins may diffuse into the cilium, but large proteins may need to associate with transport machinery to enter the cilium. (C) Potential locations for diffusion barriers preventing entrance of membrane proteins into cilia include the border between the plasma and periciliary membranes (blue), the bottom of the ciliary pocket where transition fibers anchor the basal body to the membrane (red), and the ciliary necklace (green). (D) Septin rings (red) form membrane diffusion barriers that define specific membrane compartments (green), including those of cilia, flagella, the midbody, dendritic spines, and yeast buds. (E) Soluble and membrane proteins may be retained inside cilia by directly or indirectly interacting with microtubules. (F) Selective exclusion and retention can account for differences in periciliary and plasma membrane composition. The cortical actin cytoskeleton is excluded from the region under the periciliary membrane. As a result, membrane proteins that directly or indirectly interact with actin filaments are excluded from the periciliary region. Conversely, certain proteins, such as Galectin-3, that accumulate in the periciliary region may be retained by extracytosolic interactions with the ciliary membrane or by interactions with the basal body or its associated microtubules (dashed arrows). c, cargo; GalNAc, N-acetyl-galactosamine.