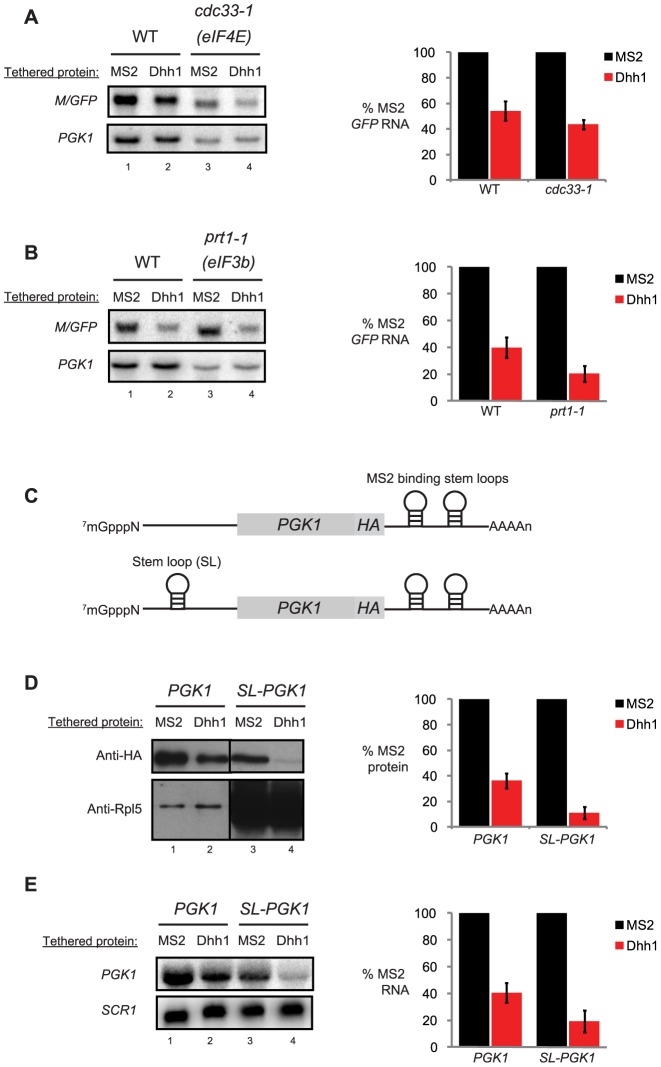
Figure 2. Tethered Dhh1 still functions under conditions in which translation initiation is limited.
(A) Northern blot analysis of steady state M/GFP levels from both wild-type and cdc33-1 (eIF4E mutant cells) cells co-expressing either MS2 alone or tethered Dhh1 grown at the restrictive temperature (37°C) for 1 h. Blots were first probed for the reporter, then were stripped and reprobed for endogenous PGK1. Relative quantitation of M/GFP signal is to the right of the gel. For a given experiment, signal with MS2 alone tethered was set to 100% and signal with Dhh1 tethered was expressed as a percentage of tethering MS2 alone. (B) Northern blot analysis of steady state M/GFP levels in both wild-type and prt1-1 (eIF3b mutant cells) cells co-expressing either MS2 alone or tethered Dhh1 grown at the restrictive temperature (37°C) for 1 h. Blots were probed and quantitated as in Figure 2A. (C) Depiction of reporter mRNAs used in Figure 2D and 2E. Both reporters are derivatives of PGK1pG and as such are under control of the GAL1 UAS; the pG tract has been replaced with two MS2 binding stem loops. Both reporters have also been engineered with an HA tag at the C-terminus of Pgk1 in order to distinguish the reporter from endogenous Pgk1 protein. The second reporter has a strong stem-loop engineered in the 5′ UTR. (D) Western blot analysis for Pgk1 and SL-Pgk1 proteins (with anti-HA) from wild-type cells co-expressing either MS2 alone or tethered Dhh1. Blots were stripped and reprobed with anti-Rpl5 antibody as a loading control. (E) Northern blot analysis for reporters in Figure 2C co-expressed with either MS2 alone or tethered Dhh1 in wild-type cells. Blots were stripped and reprobed for SCR1 as a loading control.