Figure 1.
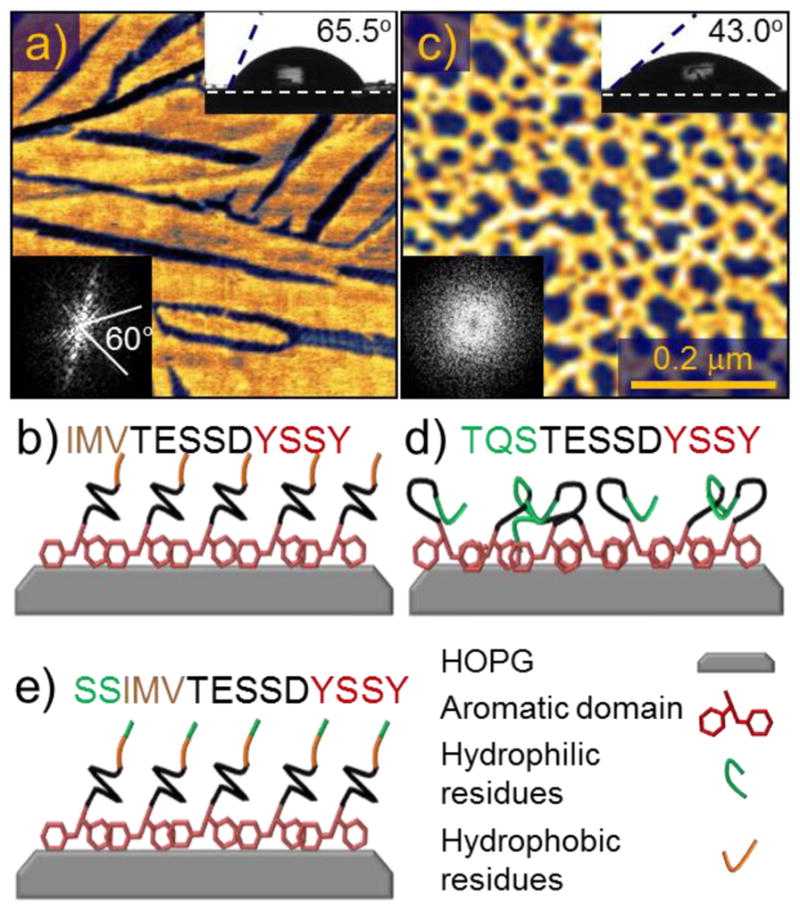
(a) Atomic force microscopy (AFM) image of the peptide GrBP5-WT on graphite with (b) Corresponding sequence (N- to C-terminus) and its schematics. (c) AFM image of GrBP5-Phil mutant on graphite with (d) Corresponding sequence and its schematics. Insets show the contact angles and the Fast Fourier Transforms (FFT) of the images that highlights the presence (spots or lines) or the lack of ordering (featureless). Schematics in (b), (d) and (e) illustrate the hypothetical conformation of the peptides within the film which produce the observed contact angles, binding through the aromatic region, and displaying either the ordered hydrophobic or disordered hydrophilic residues.
