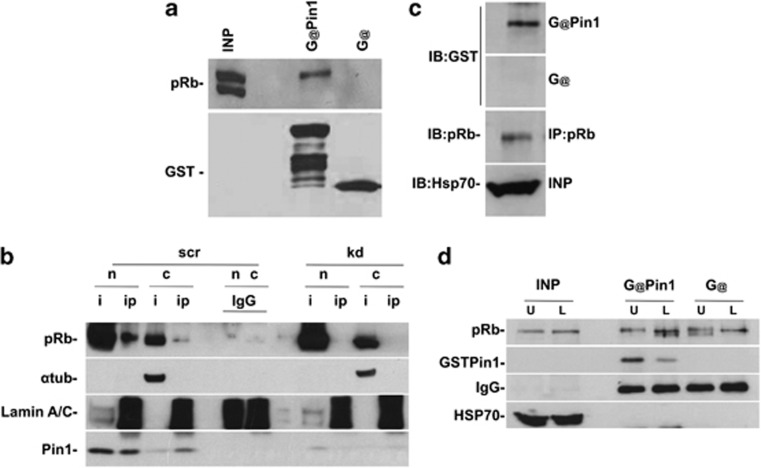
Figure 3.
In vitro and in vivo interaction between Pin1 and pRb. (a) GST-Pin1 interacts with pRb. It is noteworthy that the band corresponds to phospho-pRb. As a control, the membrane was probed with anti-GST antibody. (b) Pin1 interacts with pRb in the nucleus. Cells were immunoprecipitated with anti-Pin1 antibody, and analyzed by WB with anti-pRb antibody. As a control, PIN1 KD cells were treated as normal cells. The interaction is evident in the nucleus. α-Tubulin and lamin A/C antibodies were utilized to verify the nuclear/cytoplasmic fractions. The lamin A/C antibody yields a crossreacting nonspecific band in the IP samples, which is useful for controlling the loading of IgG lanes. n, normal; kd, PIN1 knockdown; nuc, nucleus; cyt, cytoplasm; i, input; IP, immunoprecipitation; IB, immunoblot; IGg, immunoglobulin. (c) FAR-western blot experiment showing direct interaction between Pin1 and pRb. Proteins were immunoprecipitated with anti-pRb and transferred onto a nitrocellulose membrane. Membrane was incubated with GST or GST-Pin1 (see Materials and Methods). After washing, the membrane was probed with anti-GST or anti-pRb (diluted four times) as a control. (d) Denaturing IP, which demonstrates direct interaction between Pin1 and phosphorylated pRb. Lysates were treated with lambda phosphatase and immunoprecipitated with pRb antibody. The samples were incubated with GST-Pin1, washed, transferred onto the membrane, and probed with anti-GST antibody