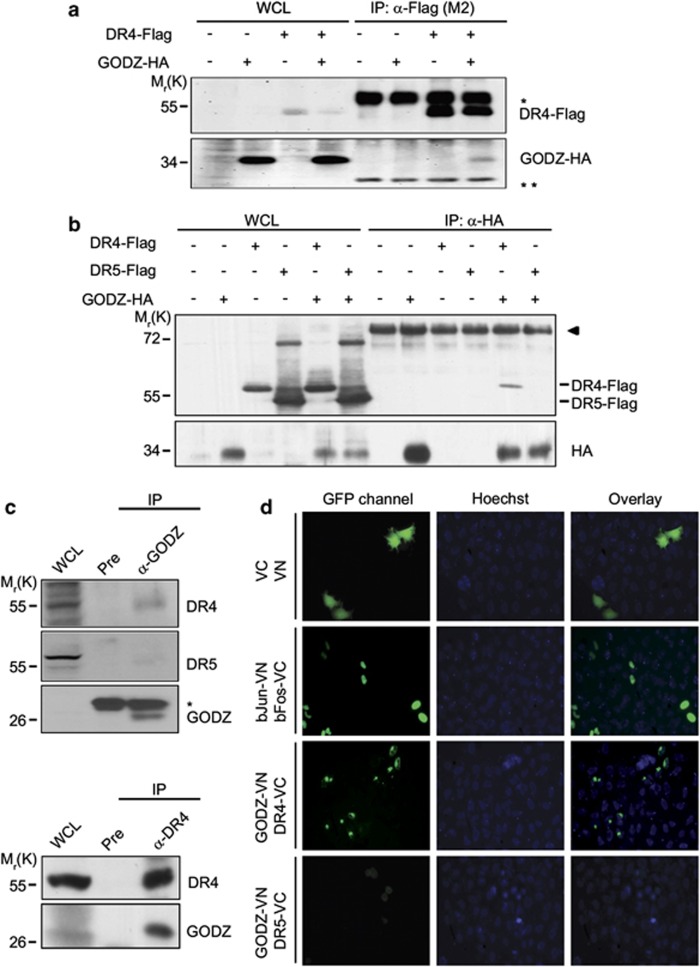
Figure 1.
GODZ binds to DR4 but not to DR5 in cells. (a and b) Reciprocal immunoprecipitation assays using HA- or Flag-tagged proteins. HEK 293T cells were transfected with pGODZ-HA, pDR4-Flag, or empty vector for 24 h and cell extracts were subjected to immunoprecipitation (IP) with anti-Flag (M2) antibody (a) or monoclonal anti-HA antibody (b) The immunoprecipitates were analyzed by western blotting using the indicated antibodies. Whole cell lysates (WCL) are indicated. Heavy chain (*) and light chain (**) of immnoglobulins were served as loading control. The arrowhead in (b) indicates nonspecific signal of the immunoprecipitates. (c) Cellular interaction between endogenous GODZ and DR4. HeLa cells extracts were prepared and IP with pre-immune serum (Pre) or anti-GODZ antibody (upper) and Pre or anti-DR4 antibody (lower). The immunoprecipitates were then analyzed by western blotting using anti-GODZ, anti-DR4, or anti-DR5 antibody. The asterisk indicates light chain (*) of immunoglobulins. (d) Visualization of the association of GODZ with DR4 in living cells using BiFC assay. HeLa cells were transiently co-transfected with equimolar amounts of pGODZ-VN and pDR4-VC, pGODZ-VN and pDR5-VC, pbJun-VN and pbFos-VC (a positive control), or pVN and pVC (a negative control). Green fluorescence images of the complementation were acquired under a fluorescence microscope (GFP; left) and then overlapped with Hoechst 33258 staining for nuclei (Hoechst, middle; and Overlay, right). The color reproduction of this figure is available at the Cell Death and Differentiation journal online