Fig. 2.
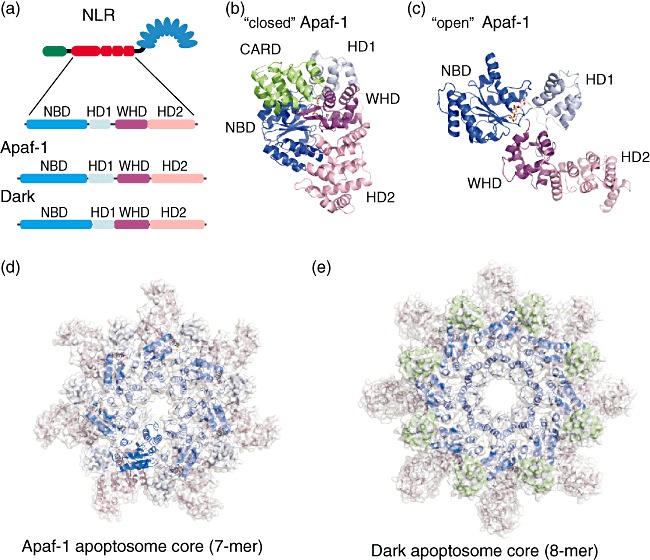
Structural organization of the apoptosome core-forming NACHT domain of human apoptotic protease-activating factor-1 (Apaf-1) and Drosophila Dark in inhibited free and active apoptosome forming stages. (a) Subdomain organization of the NACHT domain of a nucleotide-binding domain and leucine-rich repeat containing family (NLR), Apaf-1 and Dark. The NACHT domain can be subdivided into four subdomains – conserved nucleotide binding domain (NBD) and a small helical domain (HD1) that binds nucleotides, a winged helix domain (WHD) that is involved in oligomerization and a second helical domain (HD2). (b) Structure of human Apaf-1 without regulatory domains [caspase recruitment domain (CARD) and NACHT only] in the ‘close’ inactive conformation with adenosine diphosphate (ADP) bound (PDB ID 1z6t). (c) Structure of the human Apaf-1 NACHT domain with adenosine triphosphate (ATP) bound in ‘open’ active conformation capable to oligomerize (this structure was extracted from Apaf-1 apoptosome, PDB ID 3izA, chain A). (d,e) The apoptosome core structure formed by seven human Apaf-1 and 8 Drosophila Dark active NACHT domains, respectively. All subdomains are coloured according to the colouring in (a).
