Abstract
In the present study antimicrobial activity of Withania somnifera L. Dunal (Solanaceae) has been evaluated against selected pathogens. Free and bound flavonoids of different parts (root, stem, leaf and fruit) of W. somnifera have been studied for their antimicrobial activity using disc diffusion assay against three Gram negative bacteria (Escherichia coli MTCC 46, Proteus mirabilis MTCC 3310 and Pseudomonas aeruginosa MTCC 1934), one Gram positive bacteria (Staphylococcus aureus MTCC 3160) and three fungi (Candida albicans MTCC 183, Aspergillus flavus MTCC 277 and Aspergillus niger MTCC 282). Minimum inhibitory concentration (MIC) of the extracts was evaluated through micro broth dilution method, while minimum bactericidal/fungicidal concentration was determined by sub culturing the relevant samples. C. albicans was found to be the most susceptible organism followed by S. aureus, P. mirabilis, E. coli and P. aeruginosa. Out of the tested organisms, A flavus and A. niger were observed to be resistant as none of the tested extracts showed activity against them. Total activity (TA) of extracts (ml/g) against each sensitive pathogens was also evaluated. Bound flavonoid extract of root showed best activity against C. albicans (IZ 30, MIC 0.039, MFC 0.039, respectively). However all the microorganisms were found to be sensitive against the extracts tested. Total activity of bound flavonoid extract of root was found to be same for E.coli, P. mirabilis, S. aureus and C. albicans (153.84 ml/g). Results of the present study reveal that extracts of W. somnifera showing great antimicrobial potential against test microorganisms may be exploited for future antimicrobial drugs.
Keywords: Flavonoids, minimum inhibitory concentration, minimum bactericidal concentration, minimum fungicidal concentration, total activity, Withania somnifera
The plants are still widely used in ethno medicine around the world. Today, medicinal plants have recently received attention of the pharmaceutical and scientific communities and various publications have documented the therapeutic value of natural compounds in a bid to validate claims of their biological activity. Attention has been drawn to antimicrobial activity of plants and their metabolites due to the challenge of growing incidences of drug resistant pathogens. Some plants have shown the ability to overcome resistance in some organisms and this has led to researches to investigate their mechanisms of action and isolating active compounds[1]. There is a continuous and urgent need to discover new antimicrobial compounds with diverse chemical structures and novel mechanisms of action as there has been an alarming increase in the incidence of new and re-emerging infectious diseases. In the present scenario of emergence of multiple drug resistance to human pathogenic organisms, this has necessitated a search for new antimicrobial substances from other sources including plants. Higher plants produce hundreds and thousands of diverse chemicals compounds with different biological activities[2]. The antimicrobial compounds produced by plants are active against plants and human pathogenic microorganisms[3]. The substances that can either inhibit the growth of pathogens or kill them and have no or least toxicity to host cells are considered potential compounds for developing new antimicrobial drugs.
Withania somnifera, commonly known as Ashwagandha, is an important medicinal plant that has been used in Ayurvedic and indigenous medicine for over 3,000 years. In view of its varied therapeutic potential, it has also been the subject of considerable modern scientific attention. Ashwagandha roots are a constituent of over 200 formulations in Ayruvedha, Siddha and Unani medicine, which are used in the treatment of various physiological disorders[4,5]. Ashwagandha is widely claimed to have sedative, rejuvenative and life prolonging properties. It is also used as a general energy-enhancing tonic known as Medharasayana, which promotes learning and a good memory and in geriatric problems[6,7].
Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Proteus mirabilis and Candida albicans have been proved to be major causal organisms of various human infections and have been selected for the present study. P. aeruginosa is an opportunistic pathogen that causes urinary tract infections, respiratory system infections, dermatitis, soft tissue infections and a variety of systemic infections, particularly in victims of severe burns. E. coli and P. mirabilis are the culprits for human urinary tract infections[8] and most of human intestinal infections are due to the bacterium Escherichia coli, Staphylococcus aureus causes a variety of suppurative, wound infections and food poisoning in human beings. Major causative agent of nosocomial infections is Staphylococcus aureus[9] along with Escherichia coli and Proteus mirabilis, Candida albicans is notorious for causing candidiasis and candida vaginitis.
The present study investigation evaluated the antibacterial and anticandidal effects of free and bound flavonoids of Withania somnifera (root, stem, Leaf and fruits).
Different parts of W. somnifera (root, stem, leaf and fruit) were collected in the month of April from the western parts of India (Jaipur, Rajasthan). A voucher specimen has been submitted in Herbarium of Department of Botany, University of Rajasthan. Collected plant parts were separately shade dried, finely powered using a blender, and subjected to extraction following the method of Subramanian and Nagarjan, 1969. Hundred gram of each finely powered sample was Soxhlet extracted with 80% hot methanol (500 ml) on a water bath for 24 h and filtered. Each filtrate was re-extracted successively with petroleum ether (fraction I), ethyl ether (fraction II), and ethyl acetate (fraction III) using separating funnel. Petroleum ether fractions were discarded as being rich in fatty substances, whereas ethyl ether and ethyl acetate fractions were analyzed for free and bound flavonoids, respectively. Ethyl acetate fraction of each of the samples was hydrolyzed by refluxing with 7% H2SO4 for 2 h (for removal of bounded sugars from the flavonoids) and filtered. The filtrate was extracted in ethyl acetate and washed with distilled water to neutrality. Ethyl ether (free flavonoid) and ethyl acetate fractions (bound flavonoids) thus obtained were dried in vaccuo and weighed. The extracts were stored at 4° and were re- suspended in their respective solvents to get 10 mg/ml for antimicrobial assay.
Free and bound flavonoids were extracted by well established method of Subramanian and Nagarjan[10] which had also been followed by several others workers[11–14]. The extraction protocol which has been carried out in the present investigation is exclusively meant for free and bound flavonoids. In the extraction procedure ethyl ether and ethyl acetate fractions are supposed to contain free and bound flavonoids, respectively. Here bound flavonoids imply that the flavonoids that are bound with sugar moiety. These bounded sugar moieties are removed (from ethyl acetate fraction) by acid hydrolysis during extraction. If sugar part of the flavonoids is not removed, extracts do not react with spraying reagents (i.e 5% Fehling solution and 1% AlCl3 solution) during TLC analysis. On the other hand, after removal of sugar part, flavonoids become free from sugars bounded to it and now react with spraying reagent and give colour reactions during TLC analysis. Spraying reagents 5% fehling solution and 1% AlCl3 solution are exclusively used to detect flavonoids[15].
Ethyl ether fraction contains free flavonoids i.e flavonoids free from sugar moiety and during TLC shows positive colour reactions with the spraying reagents. In the ethyl ether fraction there is no need of acid hydrolysis as flavonoids in this fraction are not bound with sugar.
Test pathogenic microorganisms: P. aeruginosa (MTCC 1934), E. coli (MTCC 46), P. mirabilis (MTCC 3310), S. aureus (MTCC 3160) and C. albicans (MTCC 183), A flavus (MTCC 277) and A. niger (MTCC 282) were procured from Institute of Microbial Technology (IMTECH), Chandigarh, India. Bacterial strains were grown and maintained on Muller-Hinton Agar medium, while yeast and fungi was maintained on Sabouraud Dextrose Agar medium.
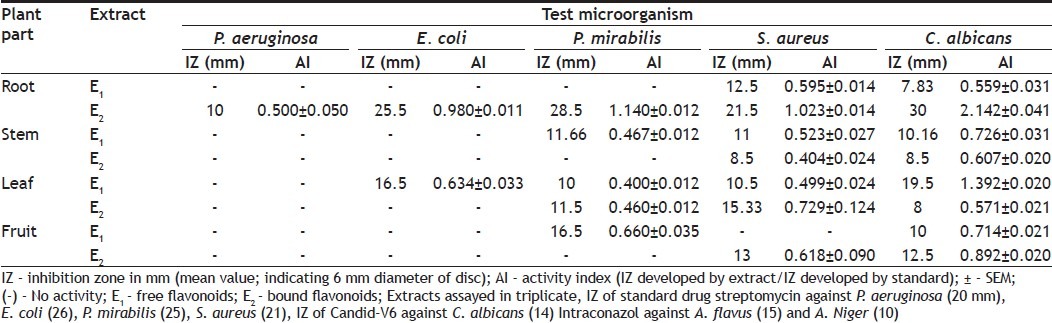
Disc diffusion assay[16] was performed for screening. Muller-Hinton agar (MHA) and Sabouraud dextrose agar (SDA) base plates were seeded with the bacterial and fungal inoculum, respectively with inoculum size 1×108 CFU/ml for bacteria and 1×107 cell/ ml for yeast). Sterile filters paper discs (Whatman no. 1, 6 mm in diameter) were impregnated with 100 μl of each of the extract (10 mg/ml) to give a final concentration of 1 mg/disc and left to dry to remove residual solvent, which might interfere with the determination. Extract discs were then placed on the seeded agar plates. Each extract was tested in triplicate with streptomycin (1 mg/disc) and candid-V6 (1 mg/ ml) as standard for bacteria and fungi, respectively. The plates were kept at 4° for 1 h for diffusion of extract, thereafter were incubated at 37° for bacteria (24 h) and 27° for fungi (48 h). Antibacterial and antifungal activity was expressed in terms of activity index (AI). Activity index for each extract was calculated (Table 1) as, Activity index=IZ produced by extract/IZ produced by standard; where IZ is inhibition zone.
TABLE 1.
ANTIMICROBIAL ACTIVITY OF WITHANIA SOMNIFERA
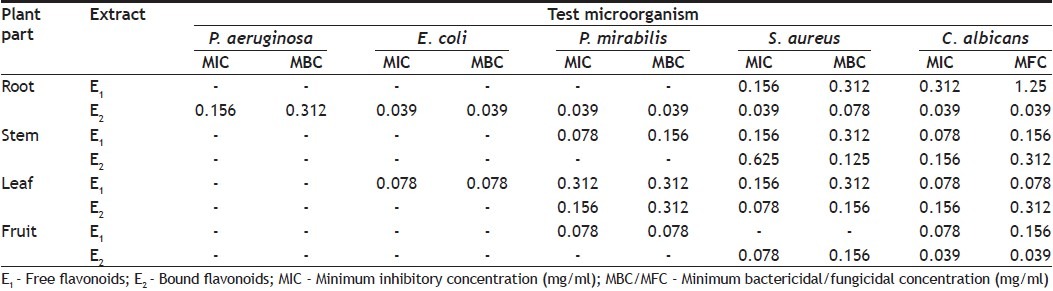
Minimum inhibitory concentration (MIC) was determined for plant extract showing antimicrobial activity against test pathogens. Broth microdilution method[17] was followed for determination of MIC values. Plant extracts were resuspended in acetone (which has no activity against test pathogens) to make 10 mg/ml final concentration and then two fold serially diluted; and added to broth media of 96-wells of microtiter plates. Thereafter 100 μl inoculum (for bacteria 1×108 CFU/ml and 1×107 CFU/ml for yeast and fungi) was added to each well. Bacterial and fungal suspensions were used as negative control, while broth containing standard drug was used as positive control. The microtiter plates were incubated at 37° for 24 h for bacteria and 28° for 48 h for yeast. Each extract was assayed in duplicate and each time two sets of microplates were prepared, one was kept for incubation while another set was kept at 4° for comparing the turbidity in the wells of micriplate. The MIC values were taken as the lowest concentration of the extracts in the well of the microtiter plate that showed no turbidity after incubation. The turbidity of the wells in the microtiter plate was interpreted as visible growth of microorganisms. The minimum bactericidal/fungicidal concentration (MBC/MFC) was determined by subculturing 50 μl from each well showing no apparent growth. Least concentration of extract showing no visible growth on subculturing was taken as MBC/MFC.
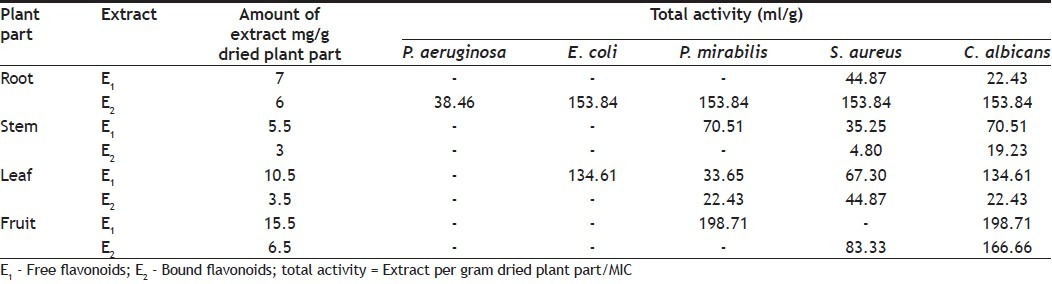
Total activity is the volume at which the test extract can be diluted with the ability to kill the microorganisms. It is calculated by dividing the amount of extract from 1 g plant material by the MIC of the same extract or compound isolated and is expressed in ml/g[18].
Antimicrobial activity (assessed in terms of inhibition zone and activity index) of the plant extracts, tested against selected microorganisms were recorded (Table 1). In the present study total eight extracts of different parts of plant were tested for their bioactivity. All 8 extracts showed significant antimicrobial activity against test microbes. Most susceptible organism in the investigation was C. albicans against which one or other all the plant extracts showed inhibition zone in some cases even more than the standard and the best activity was observed for bound flavonoids of root with IZ 30 mm, AI 2.142±0.041 and MIC 0.039 mg/ml. P. aeruginosa was found to be the most resistant microbe, against which only one extract showed activity. Best antibacterial activity against E. coli (IZ 25.5, AI 0.980±0.011, MIC 0.039), P. mirabilis (IZ 28.5, AI 1.140±0.012, MIC 0.039) and S. aureus (IZ 21.5, AI 1.023±0.014, MIC 0.039) was observed for bound flavonoids of root whereas, free flavonoids of leaf showed best activity against E. coli (IZ 16.5, AI 0.634±0.033, MIC 0.078). Free flavonoids of fruit showed bioactivity against P. mirabilis (IZ 16.5, AI 0.660±0.035, MIC 0.078) whereas, bound flavonoids showed activity against S. aureus (IZ 13, AI 0.618±0.090, MIC 0.078).
MIC and MBC/MFC values (Table 2) were evaluated for plant extracts which had shown activity in diffusion assay. The range of MIC and MBC/MFC of extracts recorded was 0.039-0.625 mg/ml and 0.039-1.25 mg/ml, respectively. In the present investigation lowest MIC value 0.039 mg/ml was recorded against E. coli, P. mirabilis, S. aureus and C. albicans whereas, against P. aeruginosa lowest MIC observed was 0.156 mg/ml, indicating significant antimicrobial potential of test extract. MIC and MBC/MFC values were found to be same for extracts of plant.
TABLE 2.
MIC AND MBC/MFC VALUES OF W. SOMNIFERA AGAINST TEST PATHOGENS
Quantity of extract obtained per gram from plant parts and total activity (TA) was calculated and recorded (Table 3). Total activity indicates the volume at which extract can be diluted without loosing ability to kill microorganism. Most of the extracts showed high values of TA against E. coli, P. mirabilis, S. aereus and C. albicans. Maximum TA values calculated were 38.46, 153.84, 198.71, 153.84 and 198.71 ml against P. aeruginosa, E. coli, P. mirabilis, S. aureus and C. albicans, respectively.
TABLE 3.
QUANTITY AND TOTAL ACTIVITY OF FREE AND BOUND FLAVONOIDS OF W. SOMNIFERA
There is a continuous and urgent need to discover new antimicrobial compounds as there is an alarming increase in the incidence of new and re-emerging infectious diseases. Medicinal plants could be a good alternative source for costly antibiotics (against which microbes are developing resistance rapidly), as most of the medicinal plants are safe with little or no side effects, cost effective and have ability to affect a wide range of antibiotic resistant microorganisms.
Present study is an effort towards this direction. In the present study IZ, AI, MIC, MBC/MFC and TA have been evaluated for each extract. For most of the extracts MIC values recorded were very low, indicating strong bio efficacy of the plant. Most of the extracts of plant were found to be potent inhibitor of tested microorganisms except P. aeruginosa, against which only one extract of the plant showed activity. Excellent activity was shown by bound flavonoids of roots having low MIC and MBC/MFC values.
Extracts with higher MBC/MFC values than MIC values against microorganisms tested, indicate the bacteriostatic/fungistatic effects of the extracts. Bound flavonoids of roots were found to be bactericidal against E. coli and P. mirabilis and fungicidal against C. albicans. Bound flavonoids of fruit were found fungicidal against C. albicans. Free flavonoids of leaf were found bactericidal against E. coli and P. mirabilis and fungicidal against C. albicans whereas, free flavonoids of fruit were found bactericidal against P. mirabilis.
Gram positive bacteria S. aureus was the second most susceptible organism after fungi C. albicans, which supported the finding that plant extracts are usually more active against Gram positive bacteria than Gram negative[19,20]. Susceptibility differences between Gram-positive and Gram-negative bacteria may be due to cell wall structural differences between these classes of bacteria. The Gram-negative bacterial cell wall outer membrane appears to act as a barrier to many substances including synthetic and natural antibiotics[21].
Boiactive potential of flavonoids have been proved by several workers[22–24]. Screening of the plant under investigation (W. somnifera) so far has not been worked out for flavonoids. Mostly the crude extracts have been screened, that too without MIC, MBC/MFC and TA determination. Such studies could only indicate their antimicrobial potential but are not helpful in establishing them as an antibiotic. In the present study IZ, AI, MIC, MBC, MFC and TA have been evaluated for each extract. For most of the extracts MIC values recorded were very low, indicating strong bio efficacy of the selected plant.
Extracts under study not only inhibit the bacterial/fungal growth but the IZ developed, was more or less permanent when compared with the IZ developed by the standard drug used, as after sometime bacterial/fungal colonies could be easily seen in IZ developed by standard drugs. In the light of the fact that microorganism are becoming resistant against the drugs in use, present investigation is of great significance, as far as the future drugs are concerned and advocates uses of selected plant by the pharmaceutical industries for preparing plant based antimicrobials drugs.
ACKNOWLEDGEMENTS
Authors are thankful to the Head of Botany Department, University of Rajasthan for providing all necessary facilities for present work. Financial assistance provided by UGC is gratefully acknowledged.
Footnotes
Singh and Kumar: Antimicrobial Efficacy of Withania Somnifera L
REFERENCES
- 1.Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr J Biotechnol. 2007;7:1797–806. [Google Scholar]
- 2.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Res. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 3.Yoganarasimhan N. Vol. 1. Bangalore: Interline Publishing Pvt Ltd; 1996. Medicinal plants of India. [Google Scholar]
- 4.Asthana R, Raina MK. Pharmacology of Withania somnifera (L.) Dunal-a review. Indian Drugs. 1989;26:199–205. [Google Scholar]
- 5.Singh S, Kumar S. Lucknow: Central Institute of Medicinal and Aromatic Plants; 1998. Withania somnifera: The Indian Ginseng Ashwagandha. [Google Scholar]
- 6.Nadkarni KM. Bombay: Popular Prakshan Limited; 1976. Indian Materia Medica; p. 1291. [Google Scholar]
- 7.Williamson EM. London: Churchill Livingstone; 2002. Major Herbs of Ayurveda; pp. 322–3. [Google Scholar]
- 8.Venier AG, Talon D, Party I, Mercier-Girard D, Bertrand X. Patient and bacterial determinants involved in symptomatic urinary tract infections caused by E.coli with and without bacteraemia. Clin Microbial Infect. 2007;13:205–8. doi: 10.1111/j.1469-0691.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez LF, Paloman M, Insausti J, Olaechea P, Cerda F, Sanchez GJ, et al. Staphylococcus aureus nosocomial infections in critically ill patients admitted in intensive care units. Med Clin (Barc) 2006;126:641–6. doi: 10.1157/13087841. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian SS, Nagarjan S. Flavonoids of the seeds of Crotolaria retusa and Crotolaria striata. Current Sci. 1969;38:65–71. [Google Scholar]
- 11.Kumar P, Khanna P. Chemical investigation of aerial plant parts and tissue culture of Solanum dulcamara Linn. Acta Botanica Indica. 1986;14:136–8. [Google Scholar]
- 12.Kumar P, Dixit VP, Khanna P. Antifertility studies of Kaempferol: Isolation and Identification from tissue culture of some medicinally important plant species. Plantes Medicinales et Phytotherapie. 1989;23:193–201. [Google Scholar]
- 13.Kumar P, Khanna P. Flavonoids from Saponarai vaccarai Linn. Indian J Plant Physiol. 1994;37:76–8. [Google Scholar]
- 14.Bhadauria S, Kumar P. Effect of plant extracts of medicinal plants against C. albicans. Flora Fauna. 1999;5:95–6. [Google Scholar]
- 15.Harborne JC. 2nd ed. London, New York: Chapman and Hall Ltd; 1984. Phytochemical Methods.A guide to modern techniques of plant analysis. [Google Scholar]
- 16.Andrews JM. BSAC standardized disc susceptibility testing method. J Antimicrob Chemother. 2001;4:43–57. doi: 10.1093/jac/48.suppl_1.43. [DOI] [PubMed] [Google Scholar]
- 17.Basri DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria antibacterial agents. Indian J Pharmacol. 2005;37:26–9. [Google Scholar]
- 18.Eloff JN. Quantifying the bioactivity of the plant extracts during screening and bioassay-guided fractionation. Phytomedicine. 2004;11:370–1. doi: 10.1078/0944711041495218. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Opake AR, Geheeb-Keller M, Hutchings AD, Terblanche SE, Jager AK. Preliminary screening of some traditional Zulu medicinal plants for antiinflammatory and antimicrobial activities. J Ethanopharmacol. 1999;68:267–74. doi: 10.1016/s0378-8741(99)00130-0. [DOI] [PubMed] [Google Scholar]
- 20.Palombo EA, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. J Ethnopharmacol. 2001;77:151–7. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 21.Tortora GJ, Funke BR, Case CL. San Francisco: Benjamin Cummings; 2001. Microbiology: An Introduction. [Google Scholar]
- 22.Ilic SB, Konstantinovic SS, Todorovic ZB. Antimicrobial activity of bioactive component from flower of Linum capitatum Kit. Facta Univ. 2004;3:73–8. [Google Scholar]
- 23.Cushnei TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandalari G, Bennett RN, Bisignano G, Trombetta D, Saija A, Faulds CB, et al. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamai Risso) peel, a by product of the essential oil industry. J Appl Microbiol. 2007;103:2050–64. doi: 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]


