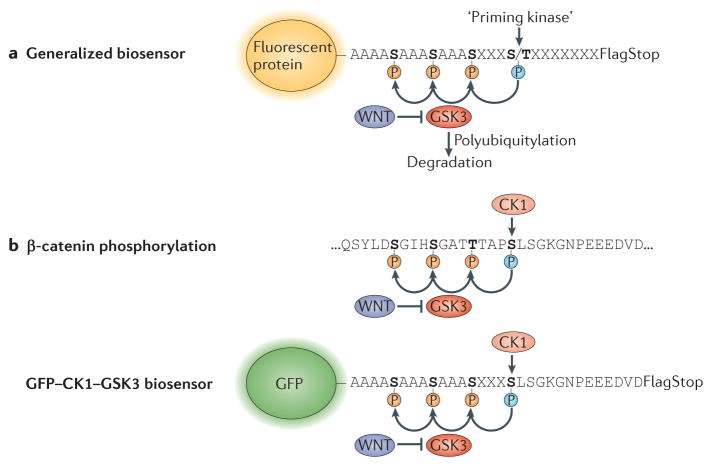
Figure 3. Generation of protein half-life biosensors and their potential use in signalling integration studies.
a | A generic biosensor of intracellular glycogen synthase kinase 3 (GSK3) activity is shown. It consists of a fluorescent protein (green fluorescent protein (GFP) or red FP (RFP)) with a carboxy-terminal peptide containing phosphorylation sites. Three artificial GSK3 sites are placed in front of a ‘priming kinase’ site (which could correspond to mitogen-activated protein kinase (MAPK), casein kinase 1 (CK1), CK2, protein kinase A (PKA) or many others); further downstream, an epitope tag (flag; which is useful for biochemical analyses) and a stop codon complete the biosensor. The presence of GSK3 phosphorylation sites in the protein destabilizes it, forming a short phosphodegron that promotes polyubiquitylation by endogenous E3 ligases and its proteasomal degradation. b | Phosphorylation sites that promote degradation in human β-catenin and in the artificial GFP biosensor GFP–CK1–GSK3, derived from their sequences12. CK1 primes three phosphorylation events by GSK3, and the presence of such priming kinase phosphorylation sites may promote specificity in signalling responses, helping to ‘insulate’ different signalling pathways from one another.