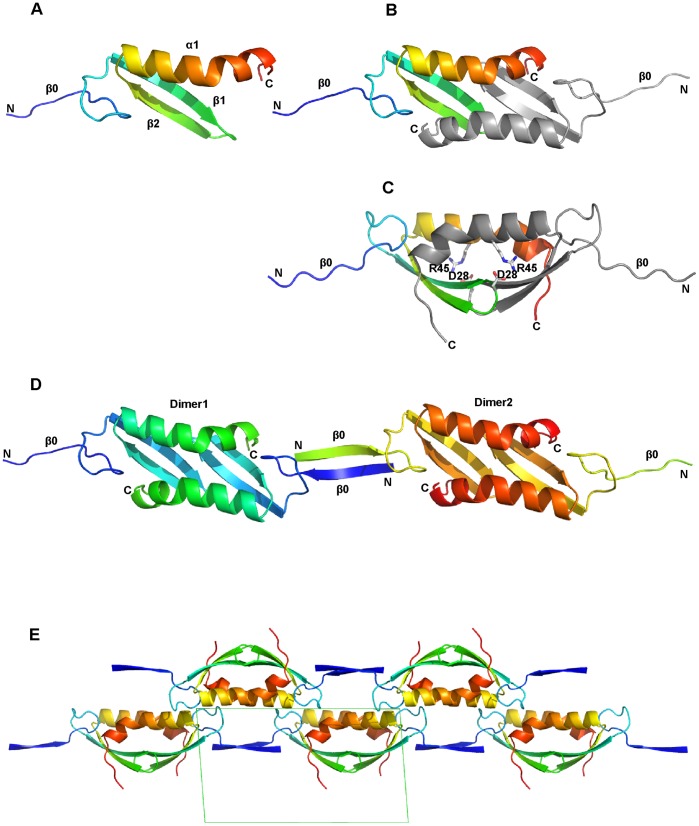
Figure 1. Cartoon diagrams of the Lsr2 N-terminal domain in the P21 crystal structure.
(A) A depiction of the monomer: the α-helix, β-strands and chain termini are labeled and the chain is colored blue-red (N-terminus to C-terminus); (B) The dimer as seen in the crystal structure, one chain is colored and the other is grey; (C) A view orthogonal to that of B showing residues critical for dimerization; (D) Lsr2 N-terminal domain oligmerization as generated by crystallographic symmetry. The N-terminus of one dimer donates one strand forming an anti-parallel β-sheet. The second strand is presented by a neighboring dimer. The β-sheet linking two dimers is shown as β0; (E) Crystallographic symmetry (in space group P21) showing the unit cell (in green) projected perpendicular to the b-axis. The two-fold screw axis generates alternating Lsr2 chains that lie back-to-back along the horizontal (in this view). For all figures protein depictions were drawn with PYMOL.