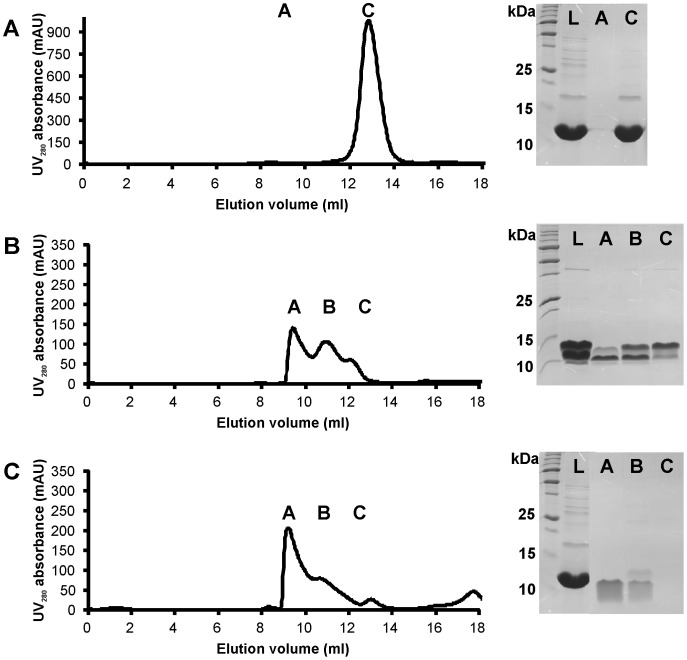
Figure 4. Addition of trypsin to N-Lsr2 facilitates oligomerization.
(A) Size exclusion chromatogram of freshly purified Lsr2 N-terminal domain showing a single dimer peak; (B) A chromatogram of the same protein construct used for crystallography (now 14 wks old) demonstrating larger oligomeric forms in solution; (C) The addition of trypsin (1∶500) for 5 min at room temperature to a fresh sample of Lsr2 N-terminal domain accelerates the formation of large oligomeric species in solution. SDS-PAGE gels showing relevant fractions from the purification: L = protein loaded on the column; A, B and C represent protein from fractions as labeled on the chromatogram. The N-terminal domain runs at a higher Mr than expected on SDS-PAGE gels due to the presence of a 6xHis-tag plus a linker.