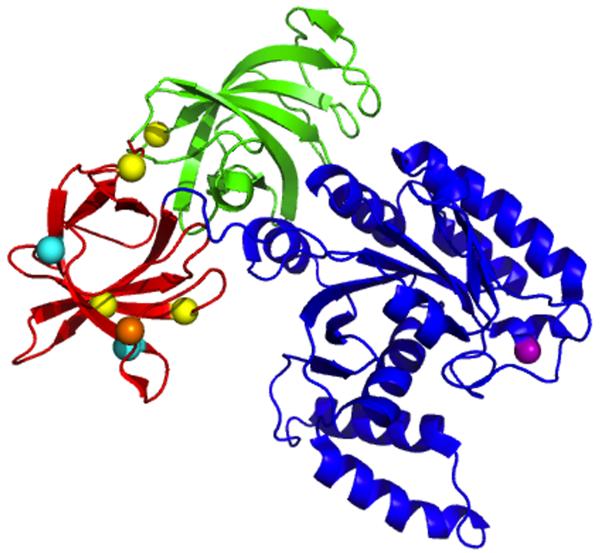
Figure 2. Location of mutations that affect non-canonical functions and known phosphorylation sites on eEF1A.
eEF1A from S. cerevisiae is composed of three well-defined domains as determined from the co-crystal structure with the C terminus of eEF1Bα. Domain I (blue) binds GTP, domain II (red) is proposed to interact with the aminoacyl end of the aa-tRNA, and domains II and III (green) are linked to actin binding and bundling. eEF1Bα binds eEF1A in domains I and II. Colored spheres indicate mutations that affect the non-canonical functions of eEF1A. In domain I, Asp156Asn (purple) affects protein turnover. In domain II, Glu286Lys and Glu291Lys (cyan) affect nuclear transport. Mutations that affect actin organization, Asn305Ser, Asn329Ser, Phe308Lys and Ser405Pro, (yellow), are shown in domains II and III. Phosphorylation of Glu298 (the yeast equivalent of human Ser300) (orange) may lead to downregulation of translation. The figure was prepared with PyMol using Protein Data Bank (PDB) 1F60.86