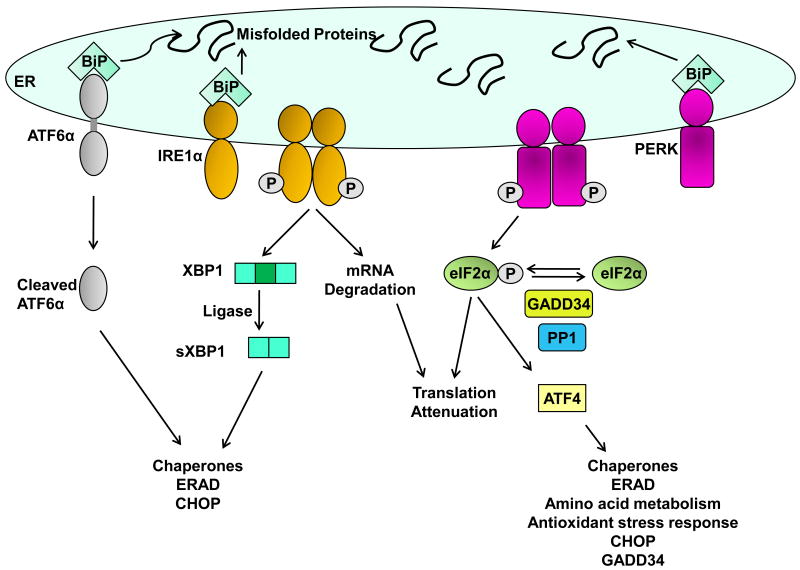
Figure 1. The unfolded protein response sensors.
Three ER membrane sensors activating transcription factor 6 α (ATF6α), inositol requiring (IRE) 1α, and PKR-like ER-localized kinase (PERK) mediate signals from the endoplasmic reticulum (ER) upon activation of the unfolded protein response (UPR). The accumulation of misfolded proteins in the ER lumen sequesters the chaperone BiP away from the lumenal domain of all three ER sensors which leads to their activation. ATF6α is activated by regulated intramembrane proteolysis in the Golgi to release the transcriptionally active 50 kDa cytosolic N-terminal domain. Cleaved ATF6α heterodimerizes with spliced XBP1 (sXBP1) to transcriptionally induce several genes encoding ER chaperones and ER-associated degradation (ERAD) proteins. IRE1α undergoes dimerization and transautophosphorylation which activates its endoribonuclease (RNase) activity. It cleaves X-box binding protein 1 (Xbp1) mRNA, which is then ligated by an uncharacterized ligase to form sXBP1 encoding a potent transcription factor, that also induces expression of ERAD proteins and chaperones. Dimerization and transautophosphorylation of PERK activates its kinase activity, leading to phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α). This leads to global translation attenuation. Selective translation of activating transcription factor 4 (ATF4) occurs following phosphorylation of eIF2α. ATF4 induces the expression of several genes including amino acid transporters, chaperones, and C/EBP homologous protein (CHOP). CHOP also induces the expression of GADD34, which associates with protein phosphatase 1 (PP1) to dephosphorylate eIF2α in a negative feedback loop, thus resuming translation.