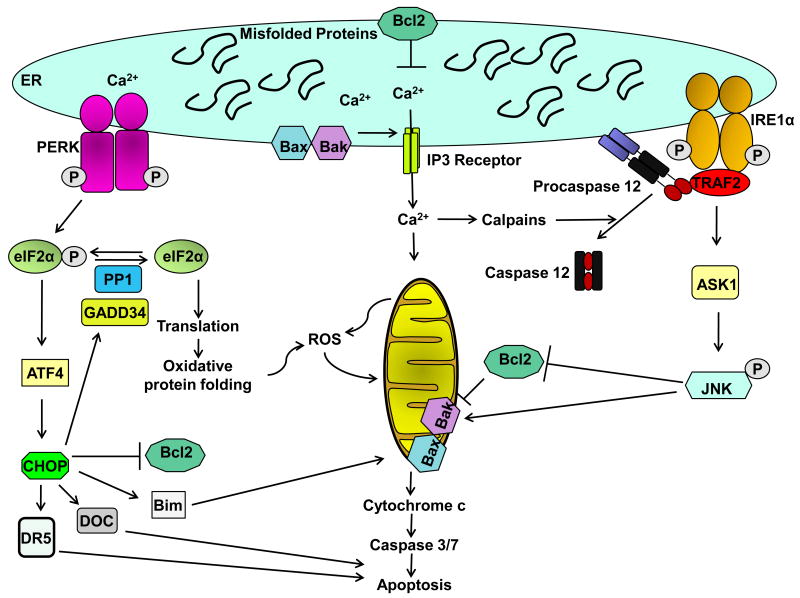
Figure 2. ER stress-induced apoptosis.
Sustained ER stress is associated with cell death. Cells that are dying upon ER stress demonstrate evidence of ongoing UPR, or a lack of resolution of the UPR. Some of the pathways that can lead to ER-stress induced apoptosis are depicted. The proapoptotic proteins Bax and Bak as well as the antiapoptotic protein Bcl-2 are localized on the ER membrane and regulate Ca2+ homeostasis. Calcium release from the ER can activate calpains, which may proteolytically activate caspase 12 to mediate apoptosis. The downstream effectors of caspase 12-induced apoptosis are not known, but presumably promote activation of terminal caspases. Calcium uptake by mitochondria leads to mitochondrial permeabilization and release of cytochrome C. CHOP can induce the expression of proapoptotic BH3-only protein Bim, the cell surface death receptor TRAIL receptor 2, other downstream of chop (DOC) mRNAs, and inhibit Bcl-2 transcription. Oxidative protein folding and mitochondrial dysfunction are associated with the accumulation of reactive oxygen species (ROS) with downstream oxidative cellular damage. JNK is activated by IRE1α via TRAF2. JNK can phosphorylate and activate proapoptotic Bcl-2 family proteins and inactivate antiapoptotic proteins.