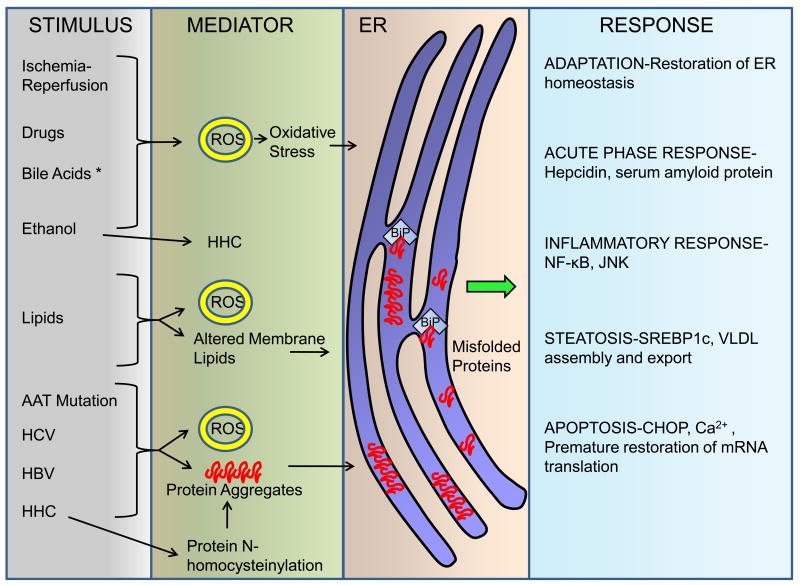
Figure 3. ER dysfunction in liver disease.
Perturbations in ER homeostasis leading to dysfunction and activation of some of the UPR sensors occur in several liver diseases. Depicted here are the stimuli that can lead to ER dysfunction. The mechanism for some of them includes generation of reactive oxygen species (ROS) leading to oxidative stress, altered membrane lipid composition, hyperhomocysteinemia (HHC) with subsequent protein N-homocysteinylation, formation of protein aggregates, or in some instances are unknown (depicted with an asterisk *). It is likely that additional mediators exist, that are yet to be characterized. In hepatocytes, ER dysfunction leads to many different responses. The UPR is activated to restore ER homeostasis. Steatosis occurs in the liver following acute ER stress, mediated by the lipid-regulatory transcription factors sterol regulatory element binding protein (SREBP)-1c and 2, as well as dysregulation of VLDL assembly and secretion. Inflammatory response cascades are activated in both acute and chronic liver diseases. In alpha-1 antitrypsin (AAT) deficiency the activation of nuclear factor-κB (NF-κB) occurs downstream of perturbations of the ER. Chronic viral hepatitis (hepatitis C virus HCV, hepatitis B virus HBV) is also associated with ER dysfunction. Sustained ER stress leads to apoptosis which may be mediated by C/EBP homologous protein (CHOP), altered Ca2+ homeostasis, and premature resumption of mRNA translation.