Abstract
Among thirty four endophytic fungal strains screened for in vitro antagonism, the endophytic fungus Cordyceps dipterigena was found to strongly inhibit mycelial growth of the plant pathogenic fungus Gibberella fujikuroi. Two new depsidone metabolites, cordycepsidone A (1) and cordycepsidone B (2), were isolated from the PDA culture extract of C. dipterigena and identified as being responsible for the antifungal activity. Elucidation of their chemical structures was carried out using 1D and 2D NMR spectroscopy in combination with IR and MS spectroscopic data. Cordycepsidone A displayed strong and dose-dependent antifungal activity against the plant pathogenic fungus Gibberella fujikuroi. The isolates were inactive in bioassays for malaria (Plasmodium falciparum), leishmaniasis (Leishmania donovani), Chagas’s disease (Trypanosoma cruzi), and cytotoxicity at 10 μg/mL. The compounds were also found to be inactive against several bacterial strains at 50 μg/mL.
Keywords: Fungal metabolite, Cordycepsidone, Cordyceps dipterigena, Gibberella fujikuroi, Antifungal activity
The International Cooperative Biodiversity Group program in Panama (ICBG-Panama) has been investigating cytotoxic, anti-parasitic and anti-microbial agents from various natural sources such as plants,1 marine organisms,2 and more recently, endophytic fungi.3,4 From a total of 3582 endophytic fungal extracts, a number of active materials have been identified in our cytotoxic or anti-parasitic drug screening. The endophytic strains were screened for identification of antifungal metabolites using antagonism assay methods5 in which the endophytic strains were grown together with a fast growing phytopathogen. Thirty-four endophytic strains were screened against the well known phytopathogen Gibberella fujikuroi. This phytopathogen devastates rice crops by causing ‘bakanae’ disease (from the Japanese ‘foolish seedling’),6 a condition which results from over-production of the plant growth hormone gibberellic acid.
Unfortunately, some higher yielding strains of rice in Asia, Africa and North America are very susceptible to infection by this pathogen. After a series of antagonistic screens on potato dextrose agar media of endophytic fungi, the most potent strain was found to be Cordyceps dipterigena7 (strain F0307) with an inhibition zone diameter of 13 mm after seven days. The crude extract from the fungus inhibited the phytopathgen at 20 μg in a disk agar diffusion assay. On the basis of this activity the fungus was grown on a larger scale to identify its active secondary metabolites. Herein, we describe the isolation8 and structural elucidation of two new antifungal depsidone compounds, cordycepsidone A (1) and cordycepsidone B (2), as the active metabolites from the extract. The new compounds were also evaluated for their anti-parasitic, anti-bacterial and cytotoxic activities.
Compound 1 was obtained as a white amorphous powder, mp 197 °C. The molecular formula C18H12O8 was determined by APCI-HR-TOFMS (m/z 357.0606 [M + H]+). The 1H NMR spectrum of 1 showed a resonance at 10.5 for an aldehyde proton, one aromatic proton at 6.85, one oxymethylene group at 5.27, and two aromatic methyl groups at 2.42 and 2.13. The 13C NMR spectrum possessed 18 carbon signals with their number of attached protons determined from a DEPT spectrum. These were present as two methyls [δC 11.3 and 21.7], one methylene [δC 68.7], two methines [δC 118.1 and 193.5] and thirteen quaternary carbons. Two long range correlations were observed by COSY between the methyl singlet at 2.42 and the aromatic proton and the methyl singlet at 2.13 and the oxymethylene protons. These suggested that the further downshielded methyl group was ortho to the aromatic proton whereas the second methyl group was ortho to the oxymethylene. HMBC correlations observed from an aromatic proton at H 6.85 (H-8) to C-16 (δC 193.5), C-7 (δC 165.3), C-10 (δC 112.6), C-11 (δC 160.7), and C-17 (δC 21.7) allowed identification of a substructure corresponding to aromatic ring A. The positions of the substituent groups about ring A were confirmed by observing HMBC correlations from H 10.54 (H-16) and H 2.42 (H-17) to various carbon resonances. Subsequently, a second substructure corresponding to aromatic ring C was identified by observing HMBC correlations from the oxymethylene at 5.27 (H-15) to C-1 (δC 168.6), C-2 (δC 109.6), C-3 (δC 146.4), C-14 (δC 145.1), C-13 (δC 114.7), and C-12 (δC 148.1) as well as from the methyl group at 2.13 (H-18) to carbons C-12 (δC 148.1), C-13 (δC 114.7), C-14 (δC 138.9) and C-4 (δC 138.9). This led to the hypothesis that aromatic ring A in 1 was similar to the lichen metabolite, diffractione A,9 whereas ring C was comparable to an endophytic fungus metabolite known as excelsione.10 A remarkable number of weak nJCH couplings (n>3), such as between H15/C5 (7JCH) and H15/C4 (5JCH), were observed in the HMBC spectra of 1. These correlations were entirely consistent with the observations made for these two related metabolites.9, 10 Therefore, structure based on the known metabolites was elucidated as shown in Fig. 1, named as cordycepsidone A.11
Figure 1.
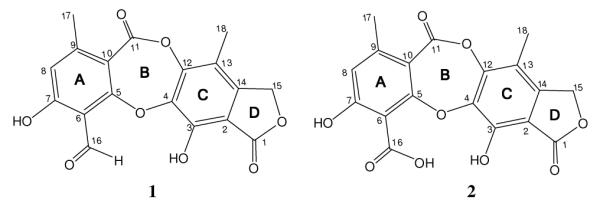
Chemical Structures of Cordycepsidone A (1) and B (2).
Compound 2 was isolated as a white amorphous powder, mp 205 °C. The molecular formula of 2 was established as C18H12O9 by APCI-HR-TOFMS (m/z 373.0563 [M + H]+), 16 mass units higher than compound 1, which suggested the addition of a single oxygen atom. The 1H NMR spectrum was very similar to that of 1, but lacked an aldehyde proton at 10.54; rather, it exhibited a proton signal corresponding to a carboxylic acid ( 14.17). This change from an aldehyde at position 16 to a carboxylic acid was the only structural difference between the two compounds. Detailed assignments for the carbon and protons in cordycepsidone B12 were accomplished by 2D NMR spectroscopic data and comparison with compound 1.
All extracts13 were subjected to spectroscopic analysis to identify any new metabolites arising from an antagonistic effect. The chemical composition of these extracts was very similar, indicating that antagonism does not have a marked effect on the secondary metabolite profile of F0307. In the paper disk assay, the extracts showed identical inhibition against phytopathogen, suggesting that the antifungal metabolite production was not induced in F0307 by the phytopathogen, but rather, is constitutively expressed. The extracts were also tested against the parasites4 Plasmodium falciparum (malaria), Leishmania donovani (leishmaniasis), and Trypanosoma cruzi (Chagas’disease), as well as MCF-7 cancer cell lines4, and found to be inactive at 10 μg/mL against all. Both compounds were inactive against several bacterial strains (Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa at 50 μg/mL).
1 and 2 belong to the depsidone class of compounds, which are more commonly associated with lichens than endophytic fungi.10, 15 It is probable that compound 2 is derived from 1 by oxidation enzymes present in the fungal strain F0307. Compound 1 showed moderate to good growth inhibitory activity against phytopathogens G. fujikuroi (MIC, 8.3 μg/mL) and Pythium ultimum (MIC, 1.2 μg/mL), but was less potent (>50) against the G. fujikuroi anamorph Fusarium subglutinans (Table 1). However, compound 2 showed a general reduction in activity in the antifungal assays, indicating the importance of the aldehyde functionality to the biological properties of compound 1. It is noteworthy that both new depsidones were highly selective against fungi and not the other assay organisms tested.
Table 1.
| Compound | Giberella fujikuroi | Pythium ultimum |
|---|---|---|
| 1 | 8.3 ± 2.7 | 1.2 ± 0.3 |
| 2 | >50 | 25.0 ± 0.1 |
| Cycloheximide | 0.39 ± 0.1 | 0.65 ± 0.2 |
The experiments were performed in triplicate and the average values were expressed.
Overall, the results presented here suggest that depsidone 1, produced by the endophytic fungal strain C. diptergena, is responsible for the observed antifungal activity. Considering the importance of pathogen control in food production, the antagonistic screen could be a useful and cost effective method to identifying novel chemical entities as antifungal agents.
Supplementary Material
Acknowledgments
This work was supported by US NIH grant for the International Cooperative Biodiversity Groups program (ICBG–Panama; 2 U01 TW006634-06). We express our thanks to the personnel of Panama s Autoridad Nacional del Ambiente for facilitating this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- (1).Jimenez-Romero C, Torres-Mendoza D, Ureña-Gonzales LD, Ortega-Barria E, McPhail KL, Gerwick WH, Cubilla-Rios L. J. Nat. Prod. 2007;70:1249–1252. doi: 10.1021/np070081d. [DOI] [PubMed] [Google Scholar]
- (2).Sanchez LM, Lopez D, Vesely BA, Della-Togna G, Gerwick WH, Kyle DE, Linington RG. J. Med. Chem. 2010;53:4187–4197. doi: 10.1021/jm100265s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Martinez-Luis S, Della-Tonga G, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios L. J. Nat. Prod. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Moreno U, Varughese T, Spadafora C, Arnold AE, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios L. Nat. Prod. Commun. 2011;6:835–840. and the references thereof.
- (5).Antagonism Screening Assay. A total of 34 endophytic fungal strains were screened for activity against the phytopathogen Gibberella fujikuroi. Briefly, agar plugs of 4 different fungal strains were circularly placed on each PDA plate with G. Fujikuroi (F1439) in the middle and incubated at 25 °C for four days. F1439 grew toward the inactive strains and left a visible inhibition zone where bioactivity occurred with the endophytic fungal strain. A total of seven active strains were identified from the initial screening. Subsequently, each strain was cultured individually with F1439 for 7 days at 25 °C in a second round of screening. F0307 showed the strongest activity with an inhibition zone diameter of 13 mm in the 7 day screen.
- (6).Waller JM, Brayford D. Int. J. Pest Manag. 1990;36:181–194. [Google Scholar]
- (7).Isolation and identification of fungal strain. The fungal strain was isolated on malt extract agar from a healthy, mature leaf of Desmotes incomparabilis collected in Coiba National Park, Veraguas, Panama, in September 2004. The strain was characterized as Cordyceps diterigena based on the DNA sequence of the nuclear ribosomal internal transcribed spacer region and the first 600bp of the nuclear ribosomal large subunit. A voucher specimen was deposited as plugs of agar in sterile water at the Smithsonian Tropical Research Institute, Panama (accession F0307).
- (8).Isolation of active metabolites. The F0307 metabolite production did not vary between the control and antagonism extracts. A larger-scale control experiment in 50 PDA plates was conducted to isolate the metabolite. Agar plugs were homogenized using a polytron, lyopholized and extracted with EtOAc (4 × 750 mL). The solvent was evaporated in vacuo, defatted with hexanes/methanol to obtain 224.7 mg of extract, which was evaluated for bioactivity against the phytopathogen at 20 μg, using the paper disk assay method. The extract was chromatographed over silica column (50 gm) using 300 mL each of hexanes (92 mg), EtOAc (37.8 mg), CHCl3-MeOH (8:2) (29.3 mg), and MeOH (69.6 mg) to obtain four fractions (B1 to B4). Compounds 1 (28.8 mg) and 2 (18.5 mg) were precipitated in MeOH from B2 and B3, respectively.
- (9).Qi HY, Jin YP, Shi YP. Chin. Chem. Lett. 2009;20:187–189. [Google Scholar]
- (10).Lang G, Cole ALJ, Blunt JW, Robinson WT, Munro MHG. J. Nat. Prod. 2007;70:310–311. doi: 10.1021/np060202u. [DOI] [PubMed] [Google Scholar]
- (11).Cordycepsidone A (1): White amorphous powder; mp 197 °C (dec); IR (nujol) νmax; 3402, 2923, 1733, 1642, 1265, 1161, 1023, 798, 757 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ10.54 (1H, s, 16-H), 6.85 (1H, s, 8-H), 5.27 (2H, s, 15-H), 2.42 (3H, s, 17-H), 2.13 (3H, s, 18-H); 13C NMR (DMSO-d6, 75 MHz) δ 193.5 (CH, C-16), 168.6 (C, C-1), 165.3 (C, C-7), 163.1 (C, C-5), 160.7 (C, C-11), 152.6 (C, C-9), 148.1 (C, C-12), 146.4 (C, C-3), 145.1 (C, C-14), 138.9 (C, C-4), 118.1 (CH, C-8), 114.7 (C, C-13), 112.6 (C, C-10), 111.5 (C, C-6), 109.6 (C, C-2), 68.7 (CH2, C-15), 21.7 (CH3, C-17), 11.3 (CH3, C-18); for HMBC and other 2D data see Supporting Information; APCI-HR-TOFMS m/z 357.0606 [M+H]+ (calcd for C18H13O8, 357.0605).
- (12).Cordycepsidone B (2): White amorphous powder; mp 205°C (dec); IR (nujol νmax; 3330, 2919, 1743, 1710, 1619, 1255, 1153, 1013, 876, 784 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ14.17 (1H, brs, 16-OH), 6.63 (1H, s, 8-H), 5.20 (2H, s, 15-H), 2.32 (3H, s, 17-H), 2.10 (3H, s, 18-H); 13C NMR (DMSO-d6, 75 MHz) δ 170.6 (C, C-16), 168.7 (C, C-1), 168.4 (C, C-7), 162.6 (C, C-5), 162.1 (C, C-11), 148.9 (C, C-3), 148.3 (C, C-12), 146.3 (C, C-9), 144.9 (C, C-14), 138.9 (C, C-4), 118.0 (CH, C-8), 111.7 (C, C-13), 110.9 (C, 10), 108.1 (C, C-2), 107.7 (C, C-6), 68.1 (CH2, C-15), 21.1 (CH3, C-17), 11.0 (CH3, C-18); for HMBC and other 2D data, see Supporting Information; APCI-HR-TOFMS m/z 373.0563 [M+H]+ (calcd for C18H13O9, 373.0554).
- (13).Extract preparation. Small scale extracts were prepared with five replicates each of F0307 (control I), F1439 (control II), and F1439/F0307 together (antagonism) at 25 °C. After seven days, a clear inhibition zone was visible on the antagonism plates and the agar was cut into three zones: the portion of the agar containing the F1439 (zone I), the portion of the agar containing F0307 (zone II), and the zone of inhibition (zone III). Thus a total of five extracts (control I, control II, and the three antagonism zones) were obtained and subjected to chemical and spectroscopic analysis.
- (14).Zgoda JR, Porter JR. Pharm. Biol. 2001;39:221–225. [Google Scholar]
- (15).Devehat FL, Tomasi S, Elix JA, Bernard A, Rouaud I, Uriac P, Boustie J. J. Nat. Prod. 2007;70:1218–1220. doi: 10.1021/np070145k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


