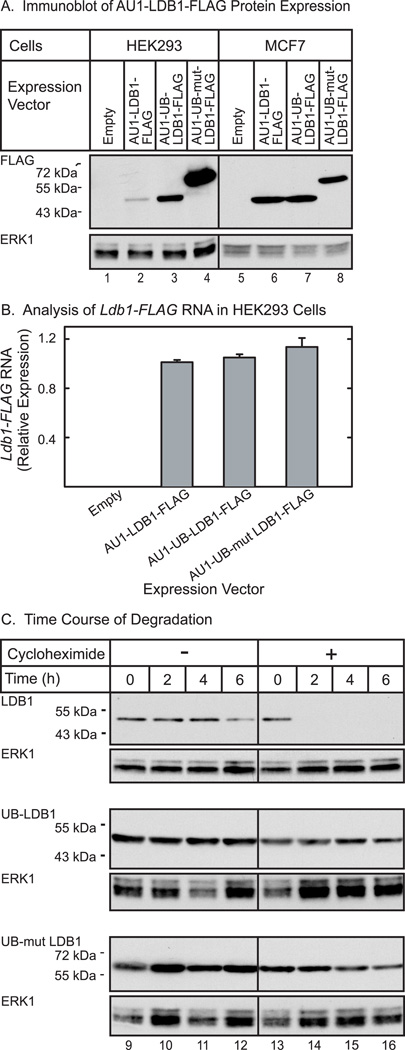
Figure 7. Ubiquitin fusion to LDB1 alters expression of the protein at a post-transcriptional level.
(A) HEK293 or MCF7 cells were transfected with an empty expression vector or an expression vector for epitope-tagged wild type, wild type ubiquitin fused, or G76V mutant ubiquitin fused LDB1. Whole cell extracts were prepared 20 h after transfection from half the cells and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody to ERK1 as a loading control. (B) RNA was prepared from half of the transfected cells described. Ldb1 and GAPDH RNA were quantitated by real-time PCR. Ldb1 RNA expression in each sample was normalized to GAPDH in each sample and then relative RNA levels were normalized so that the wild type average value for the Ldb1 group was set to 1.0. (C) HEK293 cells were transfected with an expression vector for epitope-tagged wild type, wild type ubiquitin fused, or G76V mutant ubiquitin fused LDB1. Twice as much expression vector for wild-type LDB1 was transfected in an attempt to equalize the amounts of wild type and ubiquitin fused LDB1. The next morning cells were either untreated or treated with 25 µg/ml of cycloheximide for the times indicated. Whole cell extracts were prepared and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody for ERK1 as a loading control.